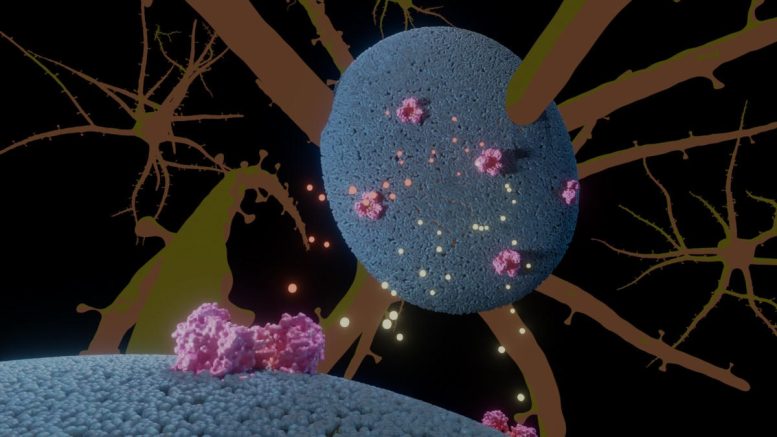
Illustration of the glutamate transporter (pink) in neural cells (blue), with glutamate and anions in yellow and orange. Credit: A. Guskov, University of Groningen
Episodic ataxia type 6 is a rare neurological disorder that affects only a small number of individuals globally. It leads to temporary loss of muscle coordination and is caused by a mutation that alters a single amino acid in a protein responsible for transporting the neurotransmitter glutamate across neural cell membranes. Scientists from the University of Groningen in the Netherlands have uncovered the mechanism by which this mutation causes malfunction in these cells. Their findings were recently published in the journal Nature Communications.
Individuals suffering from ataxia experience a loss of muscle control, which can result in difficulties with movements and speech. Among the various forms of ataxia, episodic ataxia type 6 (EA6) is a particularly rare condition, characterized by episodes of muscle control loss. Currently, only a small number of individuals, including one family in the Netherlands, have been identified as having EA6 worldwide, with the total number of known patients numbering just over a dozen.

Professor Albert Guskov, lead author of the paper in Nature Communications on the effect of a single mutation in a glutamate transport protein in neural cells. This mutation causes the neurological disease Episodic Ataxia type 6. Credit: University of Groningen
It is known that EA6 is caused by a single mutation, but how this mutation can have such a dramatic effect was thus far a mystery. ‘This protein transports glutamate across the membrane of neural cells,’ explains structural biologist Albert Guskov. The protein is inserted in the cell membrane, and the mutation changes a proline amino acid in one of the helical transmembrane domains into an arginine.
Surprise
“A proline in a helix typically causes a kink,” explains Guskov. “If a proline is changed into an arginine, we would expect this kink to disappear. To test this, we studied the structure of the mutated protein.”
Since the human transport protein is difficult to study in the lab, Guskov and his colleagues used an analogous protein from archaea, an ancient form of unicellular organism.
“This archaeal protein has been well conserved throughout evolution, and we know from previous work that it is a good model for the human transport protein, even though it transports aspartate and not glutamate,” explains Guskov.
Using cryo-electron microscopy on normal and mutated proteins placed in lipid nanodiscs, the team was able to compare the shape of the mutated protein to the normal version. In previous studies, the team had shown that part of the protein moves up and down through the membrane, much like an elevator. The hypothesis was that the mutation would cause the transmembrane kink in the protein to disappear, and that this would change the protein’s shape and block the elevator movement.
However, that was not the case. Guskov: “To our surprise, the kink was still there.”
Nevertheless, the mutation did affect the functioning of the protein. “The transport rate was reduced by a factor of two, compared to the normal protein.” Furthermore, during the transport of the aspartate, the protein transiently formed an anion channel. “And in the mutated protein, ion transport was three times higher.”
Nasty consequences
Somehow, the arginine that replaced the proline did not alter the shape of the transport protein, but it did affect its function. Therefore, the researchers performed molecular dynamics simulations, which show all the interactions of the amino acids of the protein with their surroundings. “What we noticed is that a salt bridge is formed between the arginine amino acid and the lipids of the membrane.” This salt bridge, a form of attraction between molecules, appears to slow down the movement of the elevator part of the protein.
Guskov: “If this elevator moves more slowly, it explains the decrease in aspartate transport, but it also means the transient ion channel remains open longer, thus enabling more anions to pass through.” In human neural cells, this would lead to a reduced transport of the neurotransmitter glutamate, and increased anion imbalance. These findings explain how this mutation causes ataxia. “Both have very nasty consequences for the functioning of neural cells.”
Questions
However, there is no simple way to remedy the effect of the mutation. Guskov: “Furthermore, this transporter is present throughout the body, so any drug affecting it will probably have serious side effects.” Also, since there are only a handful of patients, no drug company would invest in a cure. “Although there might be a lot more patients. Since it is an episodic illness and the symptoms can be mild, many people might not be aware of it. They are simply used to feeling unwell for a few days at a time, just like someone who suffers from migraine.”
For the scientific community, these findings raise a number of intriguing questions. Guskov: “The protein has been very well conserved throughout evolutionary history. So why did this transient anion channel appear, and has it turned out to be so beneficial for archaea that it was carried over time right to our own neurons? That is what we would like to understand.”
Reference: “Mutation in glutamate transporter homologue GltTk provides insights into pathologic mechanism of episodic ataxia 6” by Emanuela Colucci, Zaid R. Anshari, Miyer F. Patiño-Ruiz, Mariia Nemchinova, Jacob Whittaker, Dirk J. Slotboom and Albert Guskov, 31 March 2023, Nature Communications.
DOI: 10.1038/s41467-023-37503-y






Be the first to comment on "How a Single Mutation Causes a Devastating Neurological Disease"