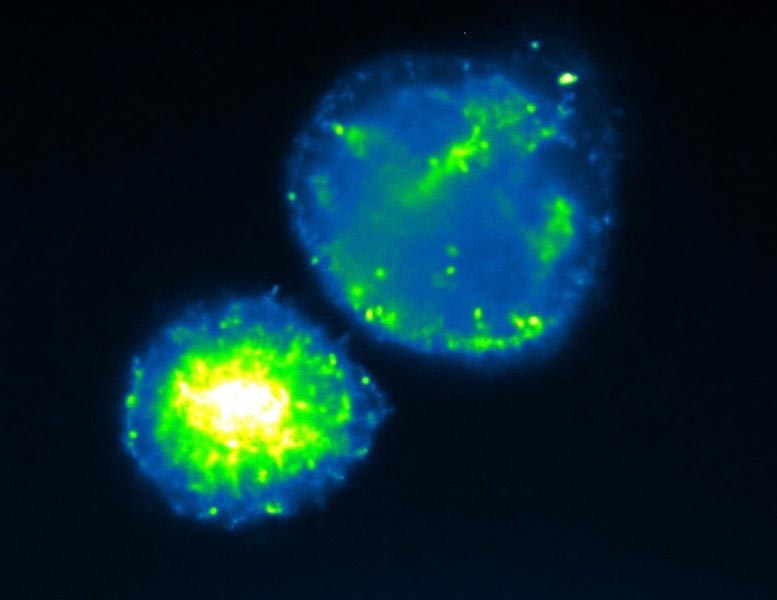
This image shows self-organized, spherical clusters of cells that decompose alginate. Differences in fluorescence indicate different metabolic states for the cells within the aggregate, a sign of the division of labor occurring during alginate decomposition. Credit: J. Schwartzman
Better Living Through Multicellular Life Cycles
For many organisms, ranging from microbes to complex multicellular life, cooperation is an essential aspect of life. It emerges when individuals share resources or partition a task in such a manner that each derives a greater benefit when acting together than they could on their own. Examples include slime mold swarming to hunt for food and reproduce, birds and fish flocking to evade predators, and bacteria forming biofilms to resist stress.
To cooperate, individuals must live in the same “neighborhood.” This neighborhood can be as small as tens of microns for bacteria. However, in environments like the ocean, it’s rare for cells with the same genetic makeup to co-occur in the same neighborhood on their own. And this necessity poses an interesting puzzle to scientists: In environments where survival hinges on cooperation, how do bacteria build their neighborhoods?
MIT professor Otto X. Cordero and colleagues took inspiration from nature to study this problem: They developed a model system based around a common coastal seawater bacterium that requires cooperation to eat sugars from brown algae. In the system, single cells were initially suspended in seawater too far away from other cells to cooperate. To share resources and grow, the cells had to find a mechanism for establishing a neighborhood. “Surprisingly, each cell was able to divide and create its own neighborhood of clones by forming tightly packed clusters,” says Cordero, associate professor in the Department of Civil and Environmental Engineering.
A new paper, published recently in the journal Current Biology, demonstrates how an algae-eating bacterium solves the engineering challenge of creating local cell density starting from a single-celled state.
“A key discovery was the importance of phenotypic heterogeneity in supporting this surprising mechanism of clonal cooperation,” says Cordero, lead author of the new paper.
Using a combination of microscopy, transcriptomics, and labeling experiments to profile a cellular metabolic state, the researchers found that cells phenotypically differentiate into a sticky “shell” population and a motile, carbon-storing “core.” The researchers propose that shell cells create the cellular neighborhood needed to sustain cooperation while core cells accumulate stores of carbon that support further clonal reproduction when the multicellular structure ruptures.
This work addresses a key piece in the bigger challenge of understanding the bacterial processes that shape our earth, such as the cycling of carbon from dead organic matter back into food webs and the atmosphere. “Bacteria are fundamentally single cells, but often what they accomplish in nature is done through cooperation. We have much to uncover about what bacteria can accomplish together and how that differs from their capacity as individuals,” adds Cordero.
Reference: “Bacterial growth in multicellular aggregates leads to the emergence of complex life cycles” by Julia A. Schwartzman, Ali Ebrahimi, Grayson Chadwick, Yuya Sato, Benjamin R.K. Roller, Victoria J. Orphan and Otto X. Cordero, 30 June 2022, Current Biology.
DOI: 10.1016/j.cub.2022.06.011
Co-authors include Julia Schwartzman and Ali Ebrahimi, former postdocs in the Cordero Lab. Other co-authors are Gray Chadwick, a former graduate student at Caltech; Yuya Sato, a senior researcher at Japan’s National Institute of Advanced Industrial Science and Technology; Benjamin Roller, a current postdoc at the University of Vienna; and Victoria Orphan of Caltech.
Funding was provided by the Simons Foundation. Individual authors received support from the Swiss National Science Foundation, Japan Society for the Promotion of Science, the U.S. National Science Foundation, the Kavli Institute of Theoretical Physics, and the National Institutes of Health.








Be the first to comment on "How an Algae-Eating Bacterium Solves a Major Environmental Engineering Challenge"