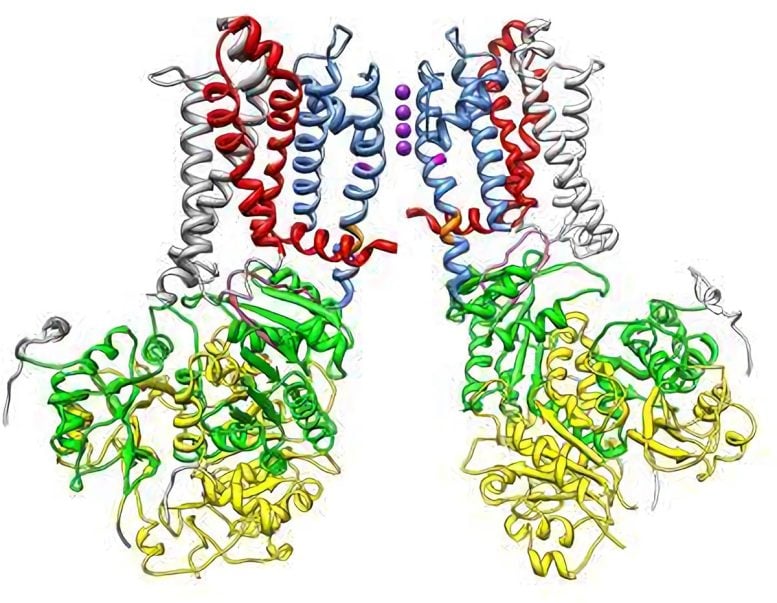
Potassium ions (shown in purple) pass through the narrow opening of a large potassium channel to generate the electrical signals that allow cells to communicate with one another. Researchers at Washington University in St. Louis have shown how an unusual protein temporarily blocks these ions from the channel, which gives cells a chance to recover so they can continue firing. Credit: Lingle laboratory
Neurobiologists at Washington University School of Medicine are studying how a protein that lacks any definable structure plays a vital role in cell channels. While looking at BK channels, the researchers found that an intrinsically disordered protein was responsible for inactivating the BK channel, challenging past notions that a protein’s precise 3-dimensional form determines its function.
Tiny pores, or channels, embedded in cell membranes are critical to the healthy functioning of cells. Charged atoms, or ions, move through these channels to generate the electrical signals that allow cells to communicate with one another.
New research at Washington University School of Medicine in St. Louis unveils some of the inner workings of certain channels involved in regulating electrical signals in nerve cells, relaxing muscle cells, and “tuning” hair cells in the inner ear.
In a report published April 22 in the advance online edition of the journal Nature, the scientists have shown how an unusual protein — one lacking any definable structure — plays a key role in temporarily blocking the movement of ions through these channels after a cell fires off an electrical signal. Preventing ions from moving through the channel is important because it gives cells time to recharge so that they can continue firing.
The researchers studied large potassium channels, called BK channels, which allow potassium ions to move in and out of cells. Looking at the channels gave the Washington University researchers an opportunity to see how so-called intrinsically disordered proteins can operate in cells.
They found that an intrinsically disordered protein was responsible for inactivating the BK channel. These proteins are of particular interest to scientists because they defy the long-held notion that a protein’s precise 3-dimensional form determines its function.
Lingle, a professor of anesthesiology and of neurobiology, and his colleagues monitored the electrical activity of BK channels as they opened and closed. Despite the disordered nature of the unstructured protein that closes the channel, the researchers found that it nestles into a receptor inside the BK channel in a highly specific way. This lock-and-key mechanism is essential to closing, or inactivating, the channel.
“It’s a two-step process, which distinguishes it from most other inactivation mechanisms that have been described,” Lingle says. “My guess is that the part of the protein that binds to the potassium channel receptor may have to move through some very narrow spaces. It may be that by having a less-defined structure, the protein can navigate more easily through tight spaces and to get to the binding site.”
Lingle and his colleagues are currently attempting to study how the channels behave in mouse cells to learn more about the physiological effects of BK channel behavior.
Problems in regulating BK channels are known to be involved in epilepsy, asthma and cardiovascular disease. A better understanding of the way those channels operate might help scientists think about new ways to treat these conditions and determine why the disordered protein domains that regulate these channels don’t have a well-defined structure.
Reference: “Stereospecific binding of a disordered peptide segment mediates BK channel inactivation” by Vivian Gonzalez-Perez, Xu-Hui Zeng, Katie Henzler-Wildman and Christopher J. Lingle, 22 April 2012, Nature.
DOI: 10.1038/nature10994
Funding for this research comes from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) and the Searle Scholars Program.








Be the first to comment on "Intrinsically Disordered Protein Responsible for Inactivating BK Channel"