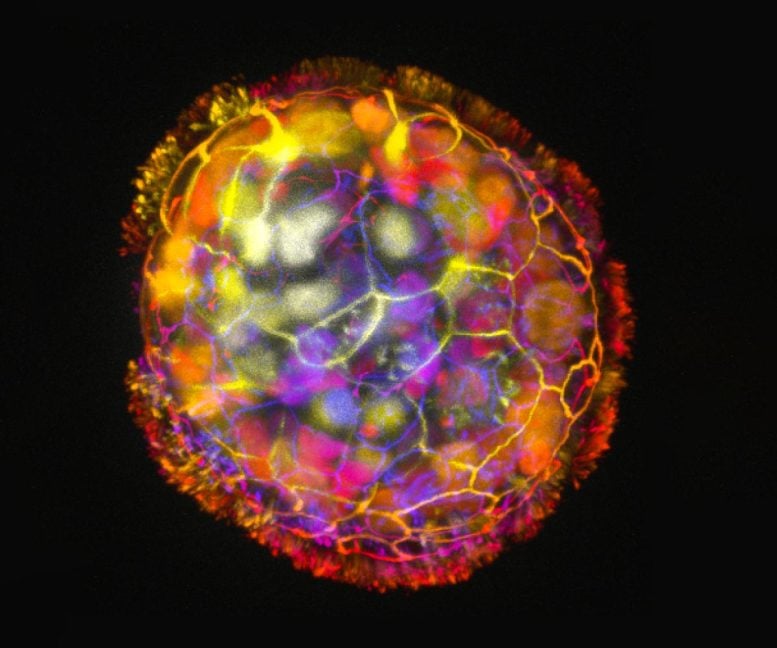
An Anthrobot is shown, depth colored, with a corona of cilia that provides locomotion for the bot. Credit: Gizem Gumuskaya, Tufts University
The multicellular bots move around and help heal “wounds” created in cultured neurons.
Researchers at Tufts University and Harvard University’s Wyss Institute have created tiny biological robots that they call Anthrobots from human tracheal cells that can move across a surface and have been found to encourage the growth of neurons across a region of damage in a lab dish.
The multicellular robots, ranging in size from the width of a human hair to the point of a sharpened pencil, were made to self-assemble and shown to have a remarkable healing effect on other cells. The discovery is a starting point for the researchers’ vision to use patient-derived biobots as new therapeutic tools for regeneration, healing, and treatment of disease.
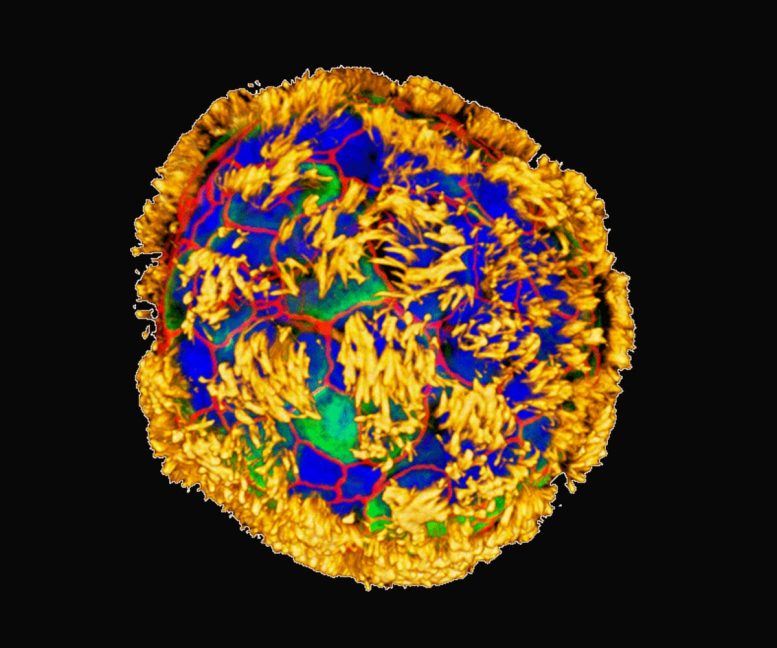
Human tracheal skin cells self-assemble into multi-cellular, moving organoids called Anthrobots. These images show Anthrobots with cilia on their surface (yellow) distributed in different patterns. Surface patterns of cilia are correlated with different movement patterns: circular, wiggling, long curves, or straight lines. Credit: Gizem Gumuskaya, Tufts University
From Xenobots to Anthrobots: A Leap in Biobotics
This advancement builds upon previous research in the laboratories of Michael Levin, Vannevar Bush Professor of Biology at Tufts University School of Arts & Sciences, and Josh Bongard at the University of Vermont in which they created multicellular biological robots from frog embryo cells called Xenobots, capable of navigating passageways, collecting material, recording information, healing themselves from injury, and even replicating for a few cycles on their own. At the time, researchers did not know if these capabilities were dependent on their being derived from an amphibian embryo, or if biobots could be constructed from cells of other species.
In the current study, published in Advanced Science, Levin, along with PhD student Gizem Gumuskaya discovered that bots can in fact be created from adult human cells without any genetic modification and they are demonstrating some capabilities beyond what was observed with the Xenobots. The discovery starts to answer a broader question that the lab has posed—what are the rules that govern how cells assemble and work together in the body, and can the cells be taken out of their natural context and recombined into different “body plans” to carry out other functions by design?
Exploring the Capabilities of Anthrobots
In this case, researchers gave human cells, after decades of quiet life in the trachea, a chance to reboot and find ways of creating new structures and tasks. “We wanted to probe what cells can do besides create default features in the body,” said Gumuskaya, who earned a degree in architecture before coming into biology. “By reprogramming interactions between cells, new multicellular structures can be created, analogous to the way stone and brick can be arranged into different structural elements like walls, archways or columns.” The researchers found that not only could the cells create new multicellular shapes, but they could move in different ways over a surface of human neurons grown in a lab dish and encourage new growth to fill in gaps caused by scratching the layer of cells.
Exactly how the Anthrobots encourage growth of neurons is not yet clear, but the researchers confirmed that neurons grew under the area covered by a clustered assembly of Anthrobots, which they called a “superbot.”
“The cellular assemblies we construct in the lab can have capabilities that go beyond what they do in the body,” said Levin, who also serves as the director of the Allen Discovery Center at Tufts and is an associate faculty member of the Wyss Institute. “It is fascinating and completely unexpected that normal patient tracheal cells, without modifying their DNA, can move on their own and encourage neuron growth across a region of damage,” said Levin. “We’re now looking at how the healing mechanism works, and asking what else these constructs can do.”
The advantages of using human cells include the ability to construct bots from a patient’s own cells to perform therapeutic work without the risk of triggering an immune response or requiring immunosuppressants. They only last a few weeks before breaking down, and so can easily be re-absorbed into the body after their work is done.
In addition, outside of the body, Anthrobots can only survive in very specific laboratory conditions, and there is no risk of exposure or unintended spread outside the lab. Likewise, they do not reproduce, and they have no genetic edits, additions, or deletions, so there is no risk of their evolving beyond existing safeguards.
How Are Anthrobots Made?
Each Anthrobot starts out as a single cell, derived from an adult donor. The cells come from the surface of the trachea and are covered with hairlike projections called cilia that wave back and forth. The cilia help the tracheal cells push out tiny particles that find their way into air passages of the lung. We all experience the work of ciliated cells when we take the final step of expelling the particles and excess fluid by coughing or clearing our throats. Earlier studies by others had shown that when the cells are grown in the lab, they spontaneously form tiny multicellular spheres called organoids.
The researchers developed growth conditions that encouraged the cilia to face outward on organoids. Within a few days they started moving around, driven by the cilia acting like oars. They noted different shapes and types of movement – the first. important feature observed of the biorobotics platform. Levin says that if other features could be added to the Anthrobots (for example, contributed by different cells), they could be designed to respond to their environment, and travel to and perform functions in the body, or help build engineered tissues in the lab.
The team, with the help of Simon Garnier at the New Jersey Institute of Technology, characterized the different types of Anthrobots that were produced. They observed that bots fell into a few discrete categories of shape and movement, ranging in size from 30 to 500 micrometers (from the thickness of a human hair to the point of a sharpened pencil), filling an important niche between nanotechnology and larger engineered devices.
Some were spherical and fully covered in cilia, and some were irregular or football-shaped with more patchy coverage of cilia, or just covered with cilia on one side. They traveled in straight lines, moved in tight circles, combined those movements, or just sat around and wiggled. The spherical ones fully covered with cilia tended to be wigglers. The Anthrobots with cilia distributed unevenly tended to move forward for longer stretches in straight or curved paths. They usually survived about 45-60 days in laboratory conditions before they naturally biodegraded.
“Anthrobots self-assemble in the lab dish,” said Gumuskaya, who created the Anthrobots. “Unlike Xenobots, they don’t require tweezers or scalpels to give them shape, and we can use adult cells – even cells from elderly patients – instead of embryonic cells. It’s fully scalable—we can produce swarms of these bots in parallel, which is a good start for developing a therapeutic tool.”
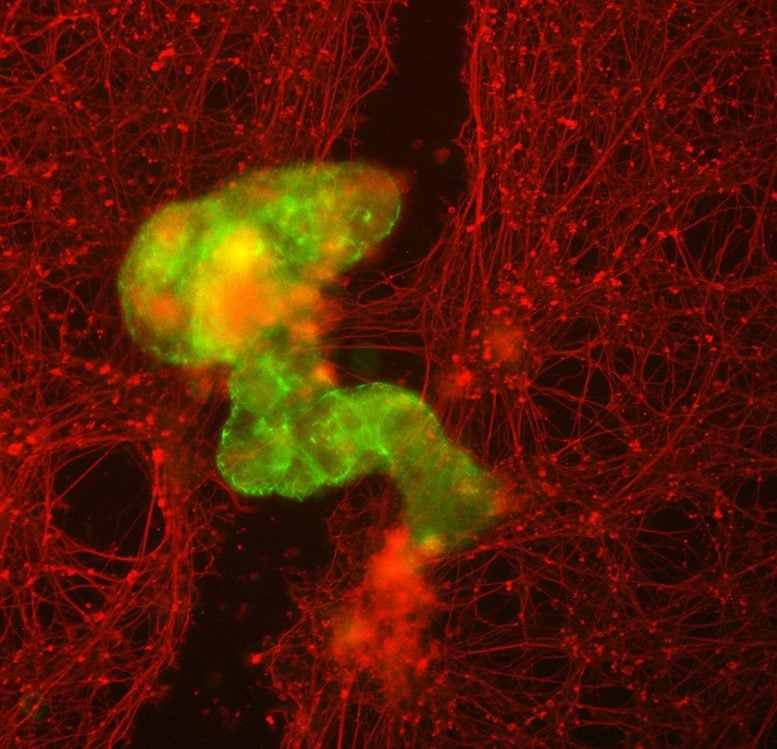
An aggregate of Anthrobots, or superbot (green), stimulates growth of neurons (red) where they had been mechanically stripped away. Credit: Gizem Gumuskaya, Tufts University
Anthrobots: The Future of Healing and Therapy
Because Levin and Gumuskaya ultimately plan to make Anthrobots with therapeutic applications, they created a lab test to see how the bots might heal wounds. The model involved growing a two-dimensional layer of human neurons, and simply by scratching the layer with a thin metal rod, they created an open ‘wound’ devoid of cells.
To ensure the gap would be exposed to a dense concentration of Anthrobots, they created “superbots” a cluster that naturally forms when the Anthrobots are confined to a small space. The superbots were made up primarily of circlers and wigglers, so they would not wander too far away from the open wound.
Although it might be expected that genetic modifications of Anthrobot cells would be needed to help the bots encourage neural growth, surprisingly the unmodified Anthrobots triggered substantial regrowth, creating a bridge of neurons as thick as the rest of the healthy cells on the plate. Neurons did not grow in the wound where Anthrobots were absent. At least in the simplified 2D world of the lab dish, the Anthrobot assemblies encouraged efficient healing of live neural tissue.
According to the researchers, further development of the bots could lead to other applications, including clearing plaque buildup in the arteries of atherosclerosis patients, repairing spinal cord or retinal nerve damage, recognizing bacteria or cancer cells, or delivering drugs to targeted tissues. The Anthrobots could in theory assist in healing tissues, while also laying down pro-regenerative drugs.
Cellular Blueprints and Regenerative Possibilities
Gumuskaya explained that cells have the innate ability to self-assemble into larger structures in certain fundamental ways. “The cells can form layers, fold, make spheres, sort and separate themselves by type, fuse together, or even move,” Gumuskaya said. “Two important differences from inanimate bricks are that cells can communicate with each other and create these structures dynamically, and each cell is programmed with many functions, like movement, secretion of molecules, detection of signals and more. We are just figuring out how to combine these elements to create new biological body plans and functions—different than those found in nature.”
Taking advantage of the inherently flexible rules of cellular assembly helps the scientists construct the bots, but it can also help them understand how natural body plans assemble, how the genome and environment work together to create tissues, organs, and limbs, and how to restore them with regenerative treatments.
Reference: “Motile Living Biobots Self-Construct from Adult Human Somatic Progenitor Seed Cells” by Gizem Gumuskaya, Pranjal Srivastava, Ben G. Cooper, Hannah Lesser, Ben Semegran, Simon Garnier and Michael Levin, 30 November 2023, Advanced Science.
DOI: 10.1002/advs.202303575









Be the first to comment on "Medical Marvel: Human Cells Transformed Into Tiny Biological Robots"