
Researchers at City of Hope have identified a crucial interaction between two proteins that facilitate cancer cell growth, offering a new target for developing effective cancer treatments. Credit: SciTechDaily.com
Scientists identify treatment target that disrupts proteins’ interaction, offering hope for new therapeutic approach.
Scientists at City of Hope, one of the largest cancer research and treatment organizations in the United States, have discovered a new cellular mechanism that plays an important role in cancer cells’ ability to cause disease. The study will be published today (February 5) in Nature Structural & Molecular Biology.
Key Protein Interaction Identified
A team led by Chun-Wei (David) Chen, Ph.D., an associate professor of systems biology at City of Hope, pinpointed two cell-surface proteins, integrin αV and β5, that partner to spur cancer cell growth. The researchers next identified a region of integrin αV called the β-propeller domain that controls the interaction between the two proteins.
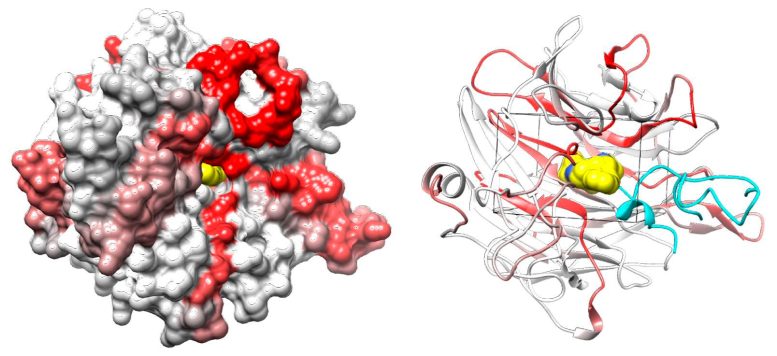
Protein surface (left) and ribbon (right) models of integrin αV’s β-propeller domain illustrate the capacity of Cpd_AV2 (yellow) in disrupting the interaction between integrin β5’s βA loop (cyan; green indicates the key interacting residue lysine 287) and integrin αV. The integrin αV’s β-propeller domain is colored by normalized CRISPR-tiling score (NCS). Credit: Chun-Wei (David) Chen, Ph.D. / City of Hope
Advancement in Cancer Treatment
Blending laboratory experiments with computer simulations, Chen’s team created a powerful digital application of CRISPR gene-tiling technology to uncover potential cancer medicines that precisely target the β-propeller domain.
After identifying the chemical compound Cpd_AV2 as a strong candidate, the team applied this compound to human cancer cells in the laboratory. Integrin αV and β5 rapidly separated, dissolving communication between the two proteins and causing cellular death, effectively halting growth in cancer cell lines.
Clinically, the researchers found integrin αV overexpression in multiple cancer types, highlighting integrin αV’s lead role in cancer progression. High levels of integrin αV were also associated with a poor prognosis in 3,700 patients with cancers of the breast, pancreas, liver, lung, and brain.
Expanding Therapeutic Horizons
By expanding opportunities for developing targeted therapies that sever the connection between integrin αV and β5, the City of Hope-led findings suggest a potent new approach for cancer treatment and future medicine discovery studies.
“Our study showcases an innovative application of CRISPR gene-tiling technology, revealing previously undiscovered sites on proteins with the capacity to cause cancer,” said Chen, who is also division director of epigenetic and transcriptional engineering at Beckman Research Institute of City of Hope. “This breakthrough opens new avenues for the advancement of next-generation oncologic therapies, marking a significant milestone in City of Hope’s battle against cancer.”
Scientists estimate that up to 1,100 different proteins live on the plasma membrane, or semi-permeable surface of the cancer cell; many of these proteins’ biological functions could influence disease progression and therapeutic response. The region offers a valuable pool of targets for medical intervention because there are plenty of surface receptors and signaling proteins that can be engineered to aid in the delivery of cancer treatments.
Integrin αV and β5 belong to the integrin family, a group of cell-surface receptors that play a central role in regulating cellular interactions, particularly across cell membranes.
Reference: “A novel class of inhibitors that disrupts the stability of integrin heterodimers identified by CRISPR-tiling-instructed genetic screens” by Nicole M. Mattson, Anthony K. N. Chan, Kazuya Miyashita, Elizaveta Mukhaleva, Wen-Han Chang, Lu Yang, Ning Ma, Yingyu Wang, Sheela Pangeni Pokharel, Mingli Li, Qiao Liu, Xiaobao Xu, Renee Chen, Priyanka Singh, Leisi Zhang, Zeinab Elsayed, Bryan Chen, Denise Keen, Patrick Pirrotte, Steven. T. Rosen, Jianjun Chen, Mark A. LaBarge, John E. Shively, Nagarajan Vaidehi, Russell C. Rockne, Mingye Feng and Chun-Wei Chen, 5 February 2024, Nature Structural & Molecular Biology.
DOI: 10.1038/s41594-024-01211-y
The research was partly supported by grants from the American Society of Hematology, Alex’s Lemonade Stand Foundation (18-11849), Stand Up To Cancer & Cancer Research UK (RT617) and the National Institutes of Health’s National Cancer Institute (CA197489, CA233691, CA236626, CA243124, CA278050, CA274649, CA233922, CA255250, CA258778), National Institute of General Medical Sciences (GM117923, CA033572). The National Cancer Institute/Developmental Therapeutics Program provided the compound library.








Be the first to comment on "Molecular Sabotage: Thwarting Cancer Growth Through Proteins"