
It is estimated that more than 6 million Americans currently have Alzheimer’s disease.
Biomarkers of misfolded proteins are found in the blood by a sensor.
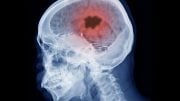
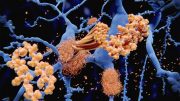
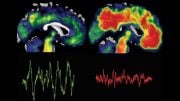
Before the first clinical symptoms appear, Alzheimer’s disease has a 15–20 year symptom-free period. A research team has discovered that it is possible to detect Alzheimer’s disease in the blood up to 17 years before any outward symptoms start to show. This is done by using an immuno-infrared sensor developed in Bochum. The sensor detects the protein biomarker amyloid-beta misfolding. As the condition progresses, this misfolding results in distinctive deposits in the brain, known as plaques.
“Our goal is to determine the risk of developing Alzheimer’s dementia at a later stage with a simple blood test even before the toxic plaques can form in the brain, in order to ensure that a therapy can be initiated in time,” says Professor Klaus Gerwert, founding director of the Centre for Protein Diagnostics (PRODI) at Ruhr-Universität Bochum. His team cooperated for the study with a group at the German Cancer Research Centre in Heidelberg (DKFZ) headed by Professor Hermann Brenner.
The team recently published the results obtained with the immuno-infrared sensor in the journal Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. This finding is backed by a comparative study that was conducted using complementary single-molecule array (SIMOA) technology and published in the same journal on March 2, 2022.
Early detection of symptom-free people with a high risk of Alzheimer’s disease
The researchers analyzed blood plasma from participants in the ESTHER study conducted in Saarland for potential Alzheimer’s biomarkers. Blood samples were collected and frozen between 2000 and 2002. The test participants were between the ages of 50 and 75 at the time and had not yet been diagnosed with Alzheimer’s disease.
The current study compared 68 people who had been diagnosed with Alzheimer’s disease during the 17-year follow-up to 240 control subjects who had not received such a diagnosis. The researchers, led by Klaus Gerwert and Hermann Brenner, wanted to see whether symptoms of Alzheimer’s disease might be detected in blood samples at the start of the study.
The immuno-infrared sensor correctly identified the 68 test individuals who eventually developed Alzheimer’s disease. For comparison, the researchers used the complementary, extremely sensitive SIMOA technology to analyze other biomarkers, specifically the P-tau181 biomarker, which is now being proposed as a potential biomarker candidate in various studies.
“Unlike in the clinical phase, however, this marker is not suitable for the early symptom-free phase of Alzheimer’s disease,” as Klaus Gerwert summarises the results of the comparative study. “Surprisingly, we found that the concentration of glial fiber protein (GFAP) can indicate the disease up to 17 years before the clinical phase, even though it does so much less precisely than the immuno-infrared sensor.”
Nonetheless, by combining amyloid-beta misfolding with GFAP concentration, the researchers were able to improve the test’s accuracy in the symptom-free stage.
Start-up aims to bring the immuno-infrared sensors to market maturity
The Bochum researchers hope that an early diagnosis based on amyloid-beta misfolding could allow Alzheimer’s drugs to be used at such an early stage that they have a substantially greater impact – for example, the drug Aduhelm, which was recently approved in the USA.
“We plan to use the misfolding test to establish a screening method for older people and determine their risk of developing Alzheimer’s dementia,” says Klaus Gerwert. “The vision of our newly founded start-up betaSENSE is that the disease can be stopped in a symptom-free stage before irreversible damage occurs.”
Despite the fact that the sensor is still under development, the technology has already been patented worldwide. BetaSENSE’s goal is to commercialize the immuno-infrared sensor and get it approved as a diagnostic device so that it may be used in clinical laboratories.
Clinical trials with Alzheimer’s drugs often fail
The drug Aduhelm, which was approved by the FDA in the United States in spring 2021, has been shown to remove amyloid-beta plaques from the brain. Previous research, however, found that it had only a minor effect on clinical symptoms such as memory loss and disorientation. As a result, the European Medicines Agency chose not to approve the medicine in Europe in the winter of 2021.
“Up to now, clinical trials for Alzheimer’s drugs have been failing by the dozen, apparently because the established plaque tests used in the trials don’t flag up the disease in time,” says Gerwert. “It seems that once plaques are deposited, they induce irreversible damage in the brain.”
To date, plaques have been detected directly in the brain using the complex and costly PET scan technology, or indirectly utilizing protein biomarker concentrations in invasively obtained cerebrospina fluid using ELISA or mass spectrometry technologies. Unlike conventional plaque diagnostics, the immuno-infrared sensor detects the earlier misfolding of amyloid-beta, which results in later plaque deposition.
“However, it is still controversially discussed whether this misfolding is the cause of Alzheimer’s disease or if it’s just an accompanying factor,” points out Gerwert. “For the therapeutic approach, this question is crucial, but it is irrelevant for the diagnosis. The misfolding indicates the onset of Alzheimer’s disease.”
“The exact timing of therapeutic intervention will become even more important in the future,” predicts Léon Beyer, first author and Ph.D. student in Klaus Gerwert’s team. “The success of future drug trials will depend on the study participants being correctly characterized and not yet showing irreversible damage at study entry.”
Biomarkers for Parkinson’s and ALS
Misfolded proteins play a central role in many neurodegenerative diseases, such as Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS). As the researchers have shown, the immuno-infrared sensor can in principle also be used to detect other misfolded proteins, such as TDP-43, which is characteristic of ALS. They don’t measure the concentration of a specific protein but detect its misfolding using disease-specific antibodies.
“Most importantly, this platform technology enables us to make a differential, precise biomarker-based diagnosis in the early stages of neurodegenerative diseases, in which the currently applied symptom-based diagnosis is very difficult and prone to errors,” stresses Gerwert.
Reference: “Amyloid-beta misfolding and GFAP predict risk of clinical Alzheimer’s disease diagnosis within 17 years” by Léon Beyer, Hannah Stocker, Dan Rujescu, Bernd Holleczek, Julia Stockmann, Andreas Nabers, Hermann Brenner and Klaus Gerwert, 19 July 2022, Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association.
DOI: 10.1002/alz.12745
Funders: Ministerium für Wissenschaft, Forschung und Kunst Baden-Württemberg, Bundesministerium für Bildung und Forschung, Bundesministerium für Familie, Senioren, Frauen und Jugend, Ministerium für Arbeit, Soziales, Frauen und Gesundheit des Saarlands, Network Aging Research Universität Heidelberg, Alzheimer Forschung Initiative, and Ministerium für Kultur und Wissenschaft des Landes Nordrhein-Westfalen.



What I’ve known about Alzheimer’s disease was caused by a species of pork parasites. It is the cause of developing amyloid plaques
Reselier my mom passed with Alzheimer’s. I’m 56 but struggle with short and long term memories. I had recently learned about detoxing parasites that can lead to Alzheimers.