The researchers also found that decreasing ATP levels enhances ClpXP (a damage-repairing enzyme)-mediated degradation of some classes of substrates.
A specific enzyme may play dual roles in cell health according to a recent study from the University of Massachusetts Amherst.
A team of researchers from the University of Massachusetts Amherst investigated the mysteries surrounding how cells handle stress in a recent study that was published in the journal Cell Reports. Researchers found that a damage-repairing enzyme known as ClpX may not only mutate to fix multiple cellular issues but can also react to shifting levels of cellular energy to maintain cell health.
“What we’re really interested in,” says Peter Chien, professor of biochemistry and molecular biology at UMass Amherst and the paper’s senior author, “is how cells respond to stress. We study a class of enzymes, called proteases, which target and destroy harmful proteins within a cell. These proteases can selectively recognize specific, individual proteins singular proteins. But how do they do this? How can they choose between healthy proteins and harmful ones?”
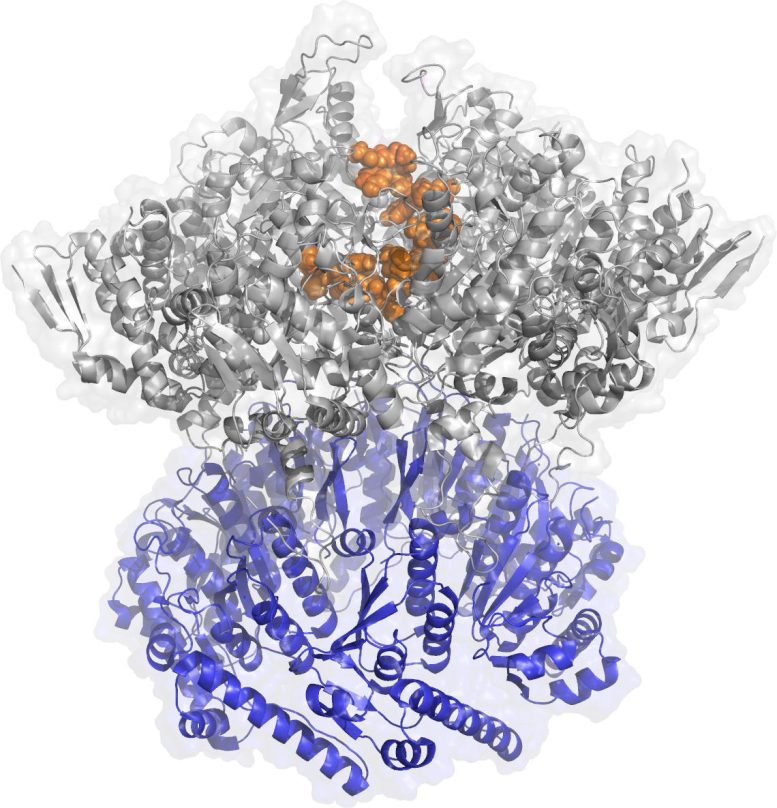
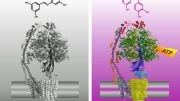
Rendering of the protease ClpX: the gray part recognizes the harmful protein, the orange grabs onto it, and the blue destroys it. Credit: Chien Lab
Chien and his co-authors focused on two specific proteases, called Lon and ClpX, each of which is finely tuned to recognize a different harmful protein, to answer this question. It had long been believed that Lon and ClpX functioned similarly to keys: each could only open one kind of lock and not another, and if a cell lacked either, severe side effects would result.
“If you’ve ever had an extremely messy college roommate,” says Chien, “you know how important it is to empty the trash regularly. Missing the Lon protease is like having a roommate who never washes, changes, or cleans.”
But following a series of experiments in which Lon was removed from bacterial cell colonies, Chien’s team saw something strange: some of the colonies were still alive.

Peter Chien (right) and UMass undergraduate researcher Oluwabusola Oreofe (left) running experiments in the Chien lab. Credit: UMass Amherst
This observation led to their first discovery: ClpX can mutate to perform a Lon-like function, though it loses some of its ClpX abilities. It’s as if, to keep your dorm-room clean, you started washing your roommate’s socks, but had to sacrifice some of your own clean laundry to do so.
In tracing out exactly how the ClpX mutation allowed the protease to expand its function, the team made its second discovery: wild, non-mutant ClpX can also perform some of Lon’s duties, under the right conditions.
It turns out that ClpX is highly sensitive to ATP, an organic compound that is the energy source for all living cells. At normal levels of ATP, ClpX focuses on its own duties, but at a specific, lower threshold it suddenly starts cleaning up after Lon.
“This is a real breakthrough in the basic understanding of how cells work,” says Chien. “It changes the rules: not only does cellular energy control how fast a cell works, but how it works, as well.”
Reference: “ATP hydrolysis tunes specificity of a AAA+ protease” by Samar A. Mahmoud, Berent Aldikacti and Peter Chien, 20 September 2022, Cell Reports.
DOI: 10.1016/j.celrep.2022.111405
The study was funded by the University of Massachusetts Amherst’s National Institutes of Health Chemistry Biology Interface Training Program, the Howard Hughes Medical Institute, the National Institutes of Health, and UMass Amherst’s Institute for Applied Life Sciences (IALS).





Be the first to comment on "New Findings Shed Light on the Mystery of How Cells Handle Stress"