
A new study found that the protein TRIM11 can inhibit neurodegeneration and improve cognitive and motor functions in animal models akin to Alzheimer’s. The research suggests TRIM11 plays a significant role in eliminating harmful protein tangles associated with neurodegenerative diseases.
Levels of the TRIM11 protein were found to be reduced in models of Alzheimer’s disease, according to new research from Penn Medicine. The study suggests that restoring the protein might improve cognitive and motor function.
New research from the Perelman School of Medicine at the University of Pennsylvania has discovered that a gene encoding a protein linked to tau production—tripartite motif protein 11 (TRIM11)—was found to suppress deterioration in small animal models of neurodegenerative diseases similar to Alzheimer’s disease (AD), while improving cognitive and motor abilities.
Furthermore, the study identified TRIM11 as a significant player in the elimination of protein tangles that lead to neurodegenerative diseases such as AD. The findings were recently published in the journal Science.
AD is the most common cause of dementia in older adults, with an estimated 6 million Americans currently living with the disease.
It is a progressive brain disorder that slowly destroys memory and thinking skills. Foundational research at Penn Medicine led by Virginia M.Y. Lee, Ph.D., the John H. Ware III Professor in Alzheimer’s Research in Pathology and Laboratory Medicine, and the late John Q. Trojanowski, MD, Ph.D., a former professor of Geriatric Medicine and Gerontology in Pathology and Laboratory Medicine, reveals that one of the underlying causes of neurodegenerative diseases is neurofibrillary tangles (NFTs) of tau proteins, which cause the death of neurons, leading to the symptoms of AD, like loss of memory.
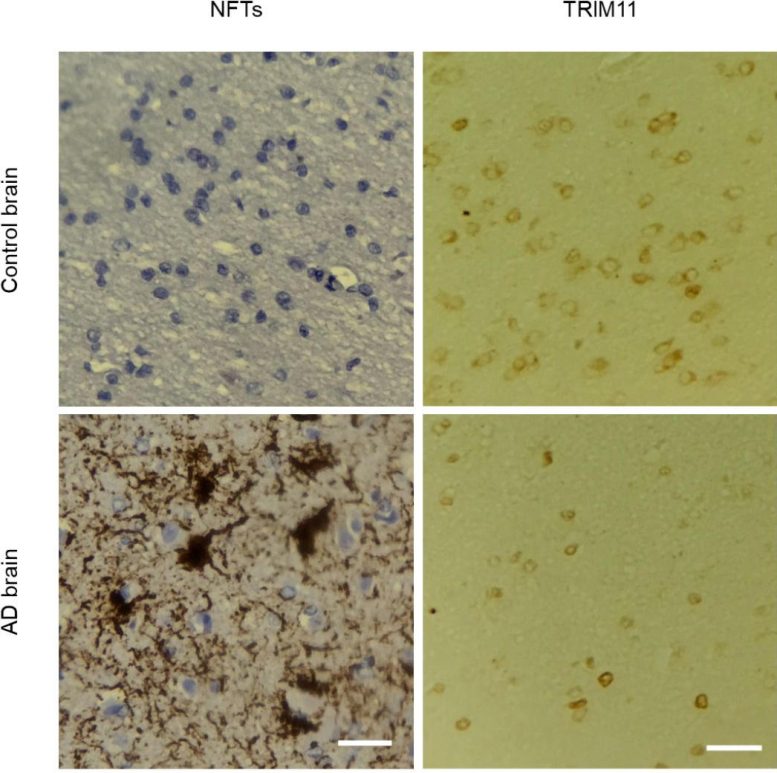
Individuals with Alzheimer’s disease (AD) have increased neurofibrillary tangles (NFTs) of tau proteins but reduced TRIM11, as shown in the bottom two quadrants, compared to individuals without AD, shown in the top two quadrants. Credit: Penn Medicine
In addition to AD, aggregation of tau proteins into NFTs is associated with over 20 other dementias and movement disorders including progressive supranuclear palsy, Pick’s disease, and chronic traumatic encephalopathy, collectively known as tauopathies. Nevertheless, how and why tau proteins clump together and form the fibrillar aggregates that makeup NFTs in patients with these diseases remains unclear. This major gap in knowledge has made the development of effective therapies challenging for researchers.
“Most organisms have protein quality control systems that remove defective proteins and prevent the misfolding and accumulation of tangles—like the ones we see with tau proteins in the brain of those with tauopathies— but until now we didn’t know how this works in humans, or why it malfunctions in some individuals and not others,” said senior author, Xiaolu Yang, Ph.D., a professor of Cancer Biology at Penn. “For the first time, we have identified the gene that oversees tau function, and have a promising target for developing treatments to prevent and slow the progression of Alzheimer’s disease and other related disorders.”
Yang and his team, including first author Zi-Yang Zhang, Ph.D., a postdoctoral researcher in Yang’s lab, previously found that TRIM proteins play an important role in protein quality control in animal cells. After examining over 70 human TRIMs, they found that TRIM11 has a major role in suppressing tau aggregation. TRIM11 possesses three main functions related to the quality control of tau proteins. First, it binds to tau proteins, especially the mutant variants that cause disease, and helps eliminate them. Second, it acts as a “chaperone” for tau, preventing the proteins from misfolding. Finally, TRIM11 dissolves pre-existing tau aggregates.
Using postmortem brain tissues of 23 individuals with AD and 14 health controls from the Center for Neurodegenerative Disease Research tissue bank—created and maintained by Lee and Trojanowski—researchers validated these findings and found that levels of TRIM11 protein are substantially reduced in the brains of individuals with AD, compared to healthy control individuals.
To determine the potential utility of TRIM11 as a therapeutic agent, researchers used adeno-associated viral vector (AAV), a tool commonly used in gene therapy, to deliver the TRIM11 gene into the brain of multiple mouse models. Researchers found that mice with tau pathologies receiving the TRIM11 gene exhibited a marked decrease in the development and accumulation of NFTs, and had much improved cognitive and motor abilities.
“Not only do these findings tell us that TRIM11 could play an important role in protecting people from Alzheimer’s and similar diseases, but we also see that we might be able to develop future therapies that replenish TRIM11 in individuals with lower levels,” said Yang. “We are eager to work with our colleagues to explore the possibility of developing gene therapies that halt the progression of neurodegenerative disease.”
Reference: “TRIM11 protects against tauopathies and is down-regulated in Alzheimer’s disease” by Zi-Yang Zhang, Dilshan S. Harischandra, Ruifang Wang, Shivani Ghaisas, Janet Y. Zhao, Thomas P. McMonagle, Guixin Zhu, Kenzo D. Lacuarta, Jianing Song, John Q. Trojanowski, Hong Xu, Virginia M.-Y. Lee and Xiaolu Yang, 28 July 2023, Science.
DOI: 10.1126/science.add6696
This study was supported by the National Institutes of Health (R01CA243520, UL1TR000003) and funding received by Penn under a sponsored research agreement with Wealth Strategy Holding Limited.
Yang is an inventor on patents and patent applications owned by the University of Pennsylvania related to TRIM proteins, and is a co-founder and equity holder of Evergreen Therapeutics LLC, which received investments from Wealth Strategy Holding Limited. Penn and Yang have either received, or may receive in the future, financial consideration related to the licensing of certain Penn intellectual property to Evergreen Therapeutics LLC.





Be the first to comment on "Scientists Discover New Promising Target for Alzheimer’s Disease Treatment"