
The research team has increased the sensitivity of the acoustic reporter genes technique to the extent that it can now image a single cell carrying an acoustic reporter gene within body tissue. Credit: Barth van Rossum for Caltech
If you are a researcher who wants to see how just a few cells in an organism are behaving, it is no simple task. The human body contains approximately 37 trillion cells; the fruit fly flitting around the overripe bananas on your counter might have 50,000 cells. Even Caenorhabditis elegans, a tiny worm commonly used in biological research, can have as many as 3,000 cells. So, how do you monitor a couple of microscopic specks amid all of that?
Scientists working in the Caltech lab of Mikhail G. Shapiro, professor of chemical engineering and Heritage Medical Research Institute Investigator, have found a way.
The new technique makes use of so-called acoustic reporter genes, of which Shapiro has been a pioneering developer. To understand acoustic reporter genes, first know that reporter genes are a specialized snippet of DNA that researchers can insert into an organism’s genome to help them understand what it is doing. Historically, reporter genes have encoded fluorescent proteins. For example, if a researcher inserts one of these reporter genes next to a gene they want to study—say, the gene that is responsible for the development of neurons—the activation of those neuron genes will also produce fluorescent protein molecules. When the right kind of light is shined upon those cells, they will light up, kind of like how a highlighter can mark a specific passage in a book.
These fluorescent reporter genes have a big disadvantage though: light does not penetrate very far through living tissues.
So, Shapiro has developed reporter genes that use sound instead of light. These genes, when inserted into a cell’s genome, cause it to produce microscopic hollow protein structures known as gas vesicles. These vesicles are normally found in certain species of bacteria that use them to stay afloat in water, but they also have the useful property of “ringing” when struck by ultrasound waves.
The idea is that when a cell producing these vesicles is imaged with ultrasound, it will send out an acoustic signal announcing its presence, allowing researchers to see where it is and what it is doing. This technique has been used to show the activity of enzymes in cells in previous work by Shapiro’s lab.
In their latest paper, the research team describes how it has increased the sensitivity of that technique so much that it can now image a single cell, located within body tissue, that is carrying an acoustic reporter gene.
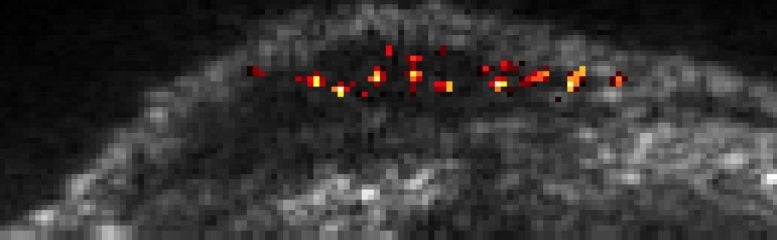
Single cells traveling through the liver of a mouse are highlighted by a new imaging technique developed in Mikhail Shapiro’s lab. Credit: Caltech/Daniel Sawyer, Shapiro Lab
“In comparison to previous work on gas vesicles, this paper allows us to see much smaller quantities of these gas vesicles,” says Daniel Sawyer (PhD ’21), lead author and former bioengineering PhD student in Shapiro’s lab. “This is like going from a satellite that can see the lights of a small town to one that can see the light from a single lamppost.”
Their improvements represent an increase of more than 1000-fold in sensitivity over the previous technique they had been using for imaging cells carrying the acoustic reporter genes. The difference lies in the ultrasound they use and how the gas vesicles respond to it.
Whereas the previous imaging technique relied on the vesicles ringing like a bell that has been struck, the new technique uses stronger ultrasound that “pops” the vesicles like a balloon.
“The vesicles produce a very strong signal in that moment,” Shapiro says. “Then the vesicles break and stop making a signal. We’re looking for the little blip.”
That blip is so clear that it can easily be detected by the researchers, even amid all the background noise produced by ultrasound penetrating through tissue. Shapiro says recent work on engineered strains of injectable bacteria that attack cancer cells, or “tumor-homing” bacteria, creates a need for better ways to track these cells to see where in the body they land. The researchers showed that when the bacteria were also engineered to carry the gas-vesicle gene, it was possible to track individual bacterial cells as they entered and traveled through the liver after being injected into the bloodstream.
Sawyer says this level of sensitivity is necessary if researchers want to use ultrasound for studying the composition of the gut microbiome, which, when disrupted, can influence conditions like Alzheimer’s disease and autism.
“There are so many species of bacteria in your gut, and some are so rare that you need something sensitive enough to see just the few of them deep inside the body,” he says.
Does popping the vesicles inside cells harm the cells? No, not really.
“The short answer is no, and the long answer is no in most practical cases,” Sawyer says. “There are some cases where single bacterial cells that are very small and have a very large amount of these gas vesicles are harmed, but it doesn’t make much of a difference to the bacterial population if a few of them become less viable. And in mammalian cells, we saw no negative effect.”
Shapiro and Sawyer are pursuing two paths for their research going forward. One path will build on what the researchers have already developed to create more advanced imaging techniques. That will involve engineering and testing new kinds of vesicles that have different properties, such as vesicles that pop more easily, or vesicles that are more robust, or smaller vesicles that can fit into places that larger vesicles cannot. The other path is finding practical applications for the technology they have developed, Sawyer says.
“In the optical microscopy field, there was this co-evolution of optical probes and microscopy methods with techniques like two-photon microscopy and light-sheet microscopy [both are types of fluorescent microscopy],” Shapiro says. “Danny’s paper is part of the development of the ultrasound analog of those imaging techniques.”
The paper describing their research, titled, “Ultrasensitive ultrasound imaging of gene expression with signal unmixing,” appears in the August 6 issue of the journal Nature Methods. Co-authors include Avinoam Bar Zion, the visitor in chemical engineering; Arash Farhadi (PhD ’20); bioengineering graduate student Shirin Shivaei; chemical engineering graduate student Bill Ling; and Audrey Lee-Gosselin, formerly of Caltech.
Reference: “Ultrasensitive ultrasound imaging of gene expression with signal unmixing” by Daniel P. Sawyer, Avinoam Bar-Zion, Arash Farhadi, Shirin Shivaei, Bill Ling, Audrey Lee-Gosselin and Mikhail G. Shapiro, 5 August 2021, Nature Methods.
DOI: 10.1038/s41592-021-01229-w
Funding for the research was provided by the National Institutes of Health.
Mikhail Shapiro is an affiliated faculty member of the Tianqiao and Chrissy Chen Institute for Neuroscience.







Be the first to comment on "Scientists Have Found a Way To “See” Single Cells Using Sound"