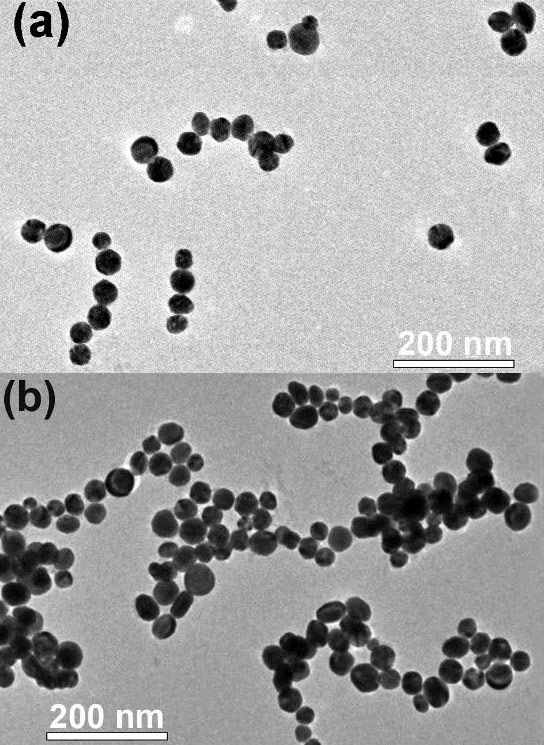
Gold nanoparticles self-assemble into long chains when bombarded with electrons. Credit: Argonne National Laboratory
Nanoscientists from the Argonne National Laboratory have viewed the self-assembly of nanoparticle chains in real-time, providing new data that could lead to new materials used to develop new, energy-relevant technologies.
In a new study performed at the Center for Nanoscale Materials at the U.S. Department of Energy’s (DOE) Argonne National Laboratory, researchers have for the first time seen the self-assembly of nanoparticle chains in situ, that is, in place as it occurs in real-time.
The scientists exposed a tiny liquid “cell” or pouch that contained gold nanoparticles covered with a positively charged coating to an intense beam of electrons generated with a transmission electron microscope. Some of the electrons that penetrated the outside of the cell became trapped in the fluid medium in the cell. These “hydrated” electrons attracted the positively charged nanoparticles, which in time reduced the intensity of charge of the positive coating.
As the hydrated electrons reduced the coating’s positive charge, the nanoparticles no longer repelled each other as strongly. Instead, their newfound relative attraction led the nanoparticles to “jump around” and eventually stick together in long chains. This self-assembly of nanoparticle chains had been detected before in different studies, but this technique allowed researchers, for the first time, to observe the phenomenon as it occurred.
“The moment-to-moment behavior of nanoparticles is something that’s not yet entirely understood by the scientific community,” said Argonne nanoscientist Yuzi Liu, the study’s lead author. “The potential of nanoparticles in all sorts of different applications and devices – from tiny machines to harvesters of new sources of energy – requires us to bring all of our resources to bear to look at how they function on the most basic physical levels.”
Self-assembly is particularly interesting to scientists because it could lead to new materials that could be used to develop new, energy-relevant technologies. “When we look at self-assembly, we’re looking to use nature as a springboard into man-made materials,” said Argonne nanoscientist Tijana Rajh, who directed the group that carried out the study.
Because the particles under study were so tiny – just a few dozen nanometers in diameter – an optical microscope would not have been able to resolve, or see, individual nanoparticles. By using the liquid cell in the transmission electron microscope at the Center for Nanoscale Materials, Liu and his colleagues could create short movies showing the quick movement of the nanoparticles as their coatings contacted the hydrated electrons.
The study, titled “In Situ Visualization of Self-Assembly of Charged Gold Nanoparticles,” was published online in the Journal of the American Chemical Society.
Reference: “In Situ Visualization of Self-Assembly of Charged Gold Nanoparticles” by Yuzi Liu, Xiao-Min Lin, Yugang Sun and Tijana Rajh, 22 February 2013, Journal of the American Chemical Society.
DOI: 10.1021/ja312620e
Funding for the research was provided by the U.S. Department of Energy’s Office of Science.








Be the first to comment on "Scientists View Self-Assembling Gold Nanoparticles in Real Time"