
New research uncovers how spin effects in iron-catalyzed hydrosilylation influence catalytic behavior, enhancing reaction rates and precision in regioselectivity. This breakthrough offers significant potential for advancing catalyst design.
Metal complex catalysts can be categorized from the standpoint of their spin states into two distinct types: closed-shell catalysts and open-shell catalysts. Closed-shell catalysts, which do not possess unpaired electrons and are commonly based on noble metals like palladium, have been more thoroughly researched and are prevalently used in industrial applications. In contrast, open-shell catalysts, characterized by their unpaired electrons and frequently derived from more abundant metals like iron, present a differing approach.
Open-shell catalysts navigate different potential energy surfaces through spin transitions, displaying catalytic behaviors markedly distinct from closed-shell catalysts. This divergence offers exciting new avenues in synthetic chemistry and is garnering increasing interest.
However, the development of open-shell catalysts is hindered by a limited understanding of their spin effects and a lack of effective control methods. Unraveling these spin effects is crucial for improving the design of crust-abundant metal catalysts and could potentially revolutionize catalysis, a prospect of significant research importance.
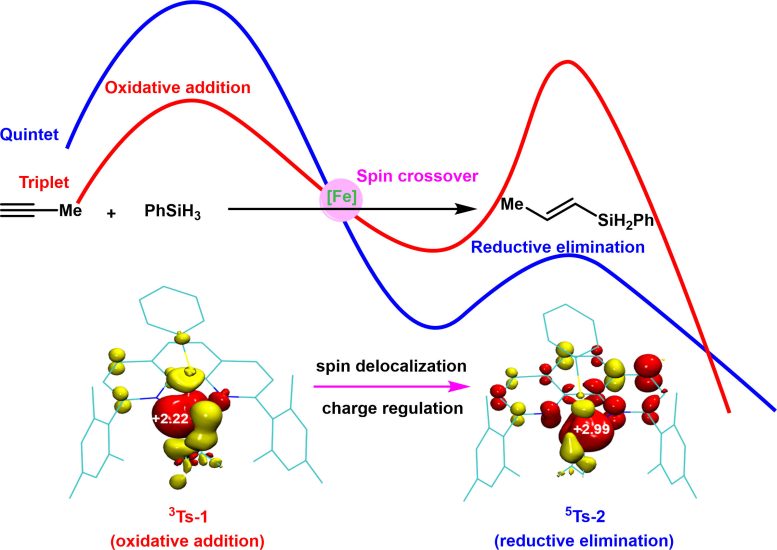
The iron-catalyzed hydrosilylation of alkynes undergoes two potential energy surfaces, the triplet (red) and quintet (blue) states, where the spin crossover effectively lowers the reaction energy barrier, and the spin-delocalization between iron and ligand dynamically modulate the oxidation and spin states of the metal center. Credit: Science China Press
To tackle these scientific challenges, Shou-Fei Zhu’s research group at Nankai University conducted a comprehensive study on the spin effects in iron-catalyzed hydrosilylation of alkynes, blending experimental work with theoretical calculations. They uncovered a novel mechanism where the spin state of open-shell iron catalysts modulates both reactivity and selectivity. These findings are recently published online in the National Science Review, with Peng He, a doctoral student at Nankai University, as the first author.
Experimental Findings and Theoretical Insights
The team synthesized a range of active iron complexes, whose structures were elucidated through X-ray single-crystal diffraction. They characterized the magnetic properties, metal valence states, and spin multiplicity of the iron center using techniques like superconducting quantum interferometry, X-ray photoelectron spectroscopy, and Mössbauer spectroscopy.
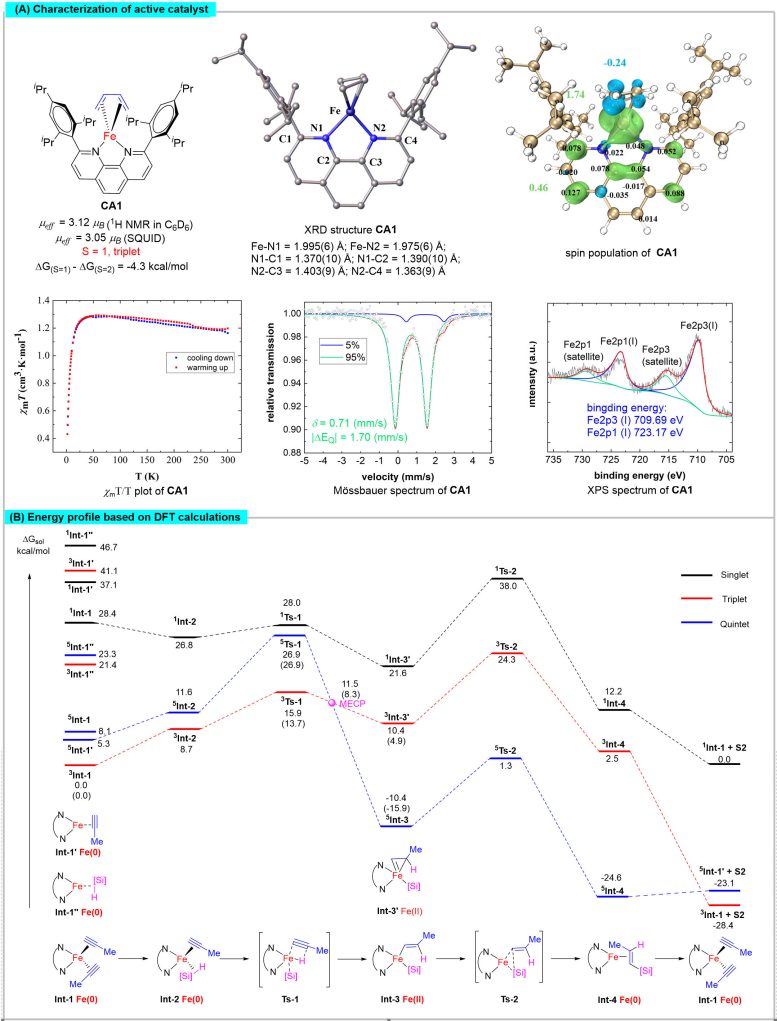
(A) Characterization of the single crystal structure and related magnetic, valence and spin states of the active catalyst and calculation of the electronic structure; (B) DFT calculations of the energy profile during the reaction. Credit: Science China Press
Theoretical calculations revealed the pivotal role of spin-delocalization interactions between iron and the 1,10-phenanthroline ligand in regulating the spin and oxidation states of the iron center. This regulation forms the structural foundation for the unique spin effects observed in iron catalysts.
Controlled experiments indicate that the reaction proceeds as a two-electron redox process, catalyzed by zero-valent iron species. These stages occur on potential energy surfaces of different spin multiplicities, with the iron catalyst facilitating transitions between these surfaces through spin crossover. This adaptability fulfills the contrasting electrostatic demands of oxidative addition and reductive elimination, significantly lowering the energy barriers of these elementary processes and thereby enhancing the reaction rate.
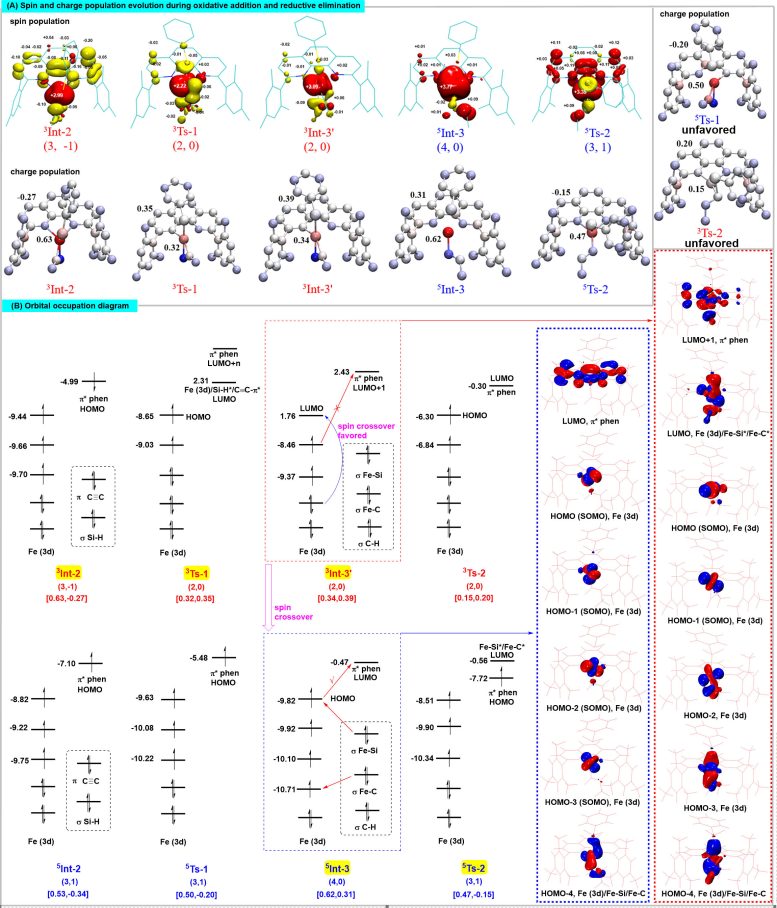
(A) Spin and charge population changes of key intermediates and transition states during the reaction process; (B) Electronic structure and orbital occupation of key intermediates and transition states during the reaction process. Credit: Science China Press
Impact on Regioselectivity and Conclusion
Spin effects also critically influence high regioselectivity. Iron catalysts adjust the spin delocalization states of complexes through specific spin states. These adjustments modulate the intramolecular noncovalent interactions within transition states, impacting their stability and enabling precise control of regioselectivity.
In summary, this study elucidates the spin effect in iron-catalyzed hydrosilylation of alkynes. The catalyst dynamically modulates the iron center’s spin and oxidation states through spin-delocalization, promoting both oxidative addition and reductive elimination processes with diametrically opposed electrostatic requirements in the catalytic cycle. Additionally, it influences regioselectivity by altering noncovalent interactions in the transition states. These insights are poised to guide the discovery and application of open-shell catalysts.
Reference: “Spin effect on redox acceleration and regioselectivity in Fe-catalyzed alkyne hydrosilylation” by Peng He, Meng-Yang Hu, Jin-Hong Li, Tian-Zhang Qiao, Yi-Lin Lu and Shou-Fei Zhu, 20 December 2023, National Science Review.
DOI: 10.1093/nsr/nwad324





Be the first to comment on "Spin State Secrets: Unlocking the Mysteries of Open-Shell Catalysts"