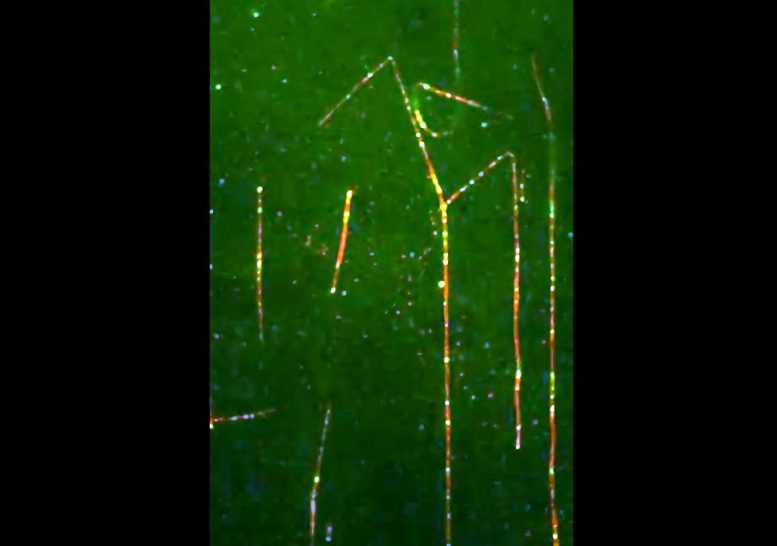
Without any hindrance, dynein moves quickly along the microtubules. Credit: Harvard Medical School

With Lis1 acting as a molecular clutch, dynein slows down on the track. Credit: Harvard Medical School
By using common baker’s yeast as a model system, Harvard researchers discovered how the key motor protein dynein interacts with a molecule called Lis1.
Molecular motors keep us alive. These cellular transport services, built from proteins, circulate essential chemical packages between the heart of the cell, the nucleus, and the cell periphery. In elongated cells such as neurons, this can be a big commute in molecular miles, equivalent to a person walking from Boston to Manhattan. The constant shuttling of materials by motors keeps cells thriving. When the process malfunctions, a host of neurological diseases can occur.
Because these motors are so critical to how life operates, researchers have been interested in taking these motors apart and learning how they work. Now, scientists in the lab of Samara Reck-Peterson, HMS assistant professor of cell biology, have discovered how the key motor protein dynein interacts with a molecule called Lis1—an interaction that, when faulty, can result in lissencephaly, or “smooth brain,” a disorder in which layers of the brain fail to form properly.
The results were published today (August 31) in the journal Cell.
For many years scientists have tried to identify precisely how dynein and Lis1 fit together. However, the sheer size of dynein has made traditional methods of analysis difficult.
Reck-Peterson, along with postdoctoral researchers Julie Huang and Anthony Roberts and collaborator Andres Leschziner, associate professor of molecular and cellular biology at Harvard University in the Department of Molecular and Cellular Biology, overcame this barrier by using common baker’s yeast as a model system. Unlike mammalian cells, yeast cells do not need dynein to survive, and thus the researchers were able to mutate and manipulate the protein at will.
“We were able to make the molecule, isolate the parts necessary for the Lis1 interaction and perform the first structural experiments,” said Reck-Peterson.
The team discovered that Lis1 does not interact with the part of dynein equivalent to its “engine,” as had previously been suspected. Instead, rather than linking to dynein’s engine, the researchers found that Lis1 acts as the motor’s “clutch.” As a molecular motor, dynein travels the cell on tracks called microtubules; long filaments that comprise the cell’s transportation infrastructure. Lis1 causes dynein to pause on the track so that it can receive cargo. The researchers believe it might also help dynein maneuver large objects.
There is still much to learn about these motors.
“We still don’t have a full understanding of how dynein is regulated,” said Huang. “We don’t know how it moves so many different things. In fact, after it gets to the end of the track, we don’t know yet how it gets recycled back.”
“To test our model and its implications for brain development, we need to study this in mammalian cells, and we’re developing the tools to do that,” said Reck-Peterson. “I’m pretty confident that when we eventually compare yeast to human cells, despite some subtle differences, the overall picture will be very similar.”
Reference: “Lis1 Acts as a “Clutch” between the ATPase and Microtubule-Binding Domains of the Dynein Motor” by Julie Huang, Anthony J. Roberts, Andres E. Leschziner and Samara L. Reck-Peterson, 31 August 2012, Cell.
DOI: 10.1016/j.cell.2012.07.022
This research is funded by the Rita Allen Foundation, The Giovanni Armenise-Harvard Foundation and a National Institutes of Health Director’s New Innovator Award (1 DP2 OD004268-01).








Be the first to comment on "Study Reveals How Lis1 Regulates Dynein Motility"