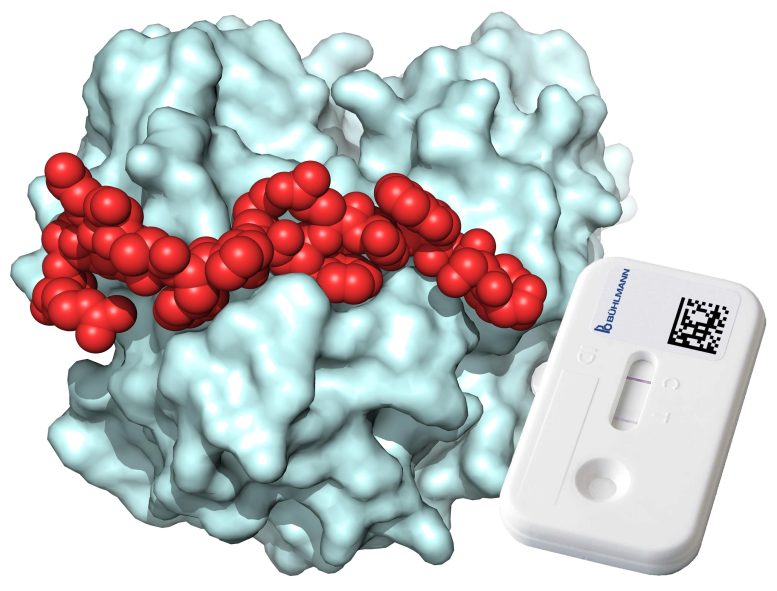
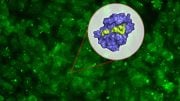
X-ray structure of the 18-amino acid peptide (red) bound to the inflammation marker calprotectin (light blue). A lateral flow cassette is shown. Credit: Christian Heinis (EPFL)/Joëlle Jourdan (BÜHLMANN AG)
Scientists from BÜHLMANN and EPFL have developed peptides as an alternative to antibodies for detecting the protein calprotectin, crucial for diagnosing and monitoring inflammatory disorders. These peptides offer a more accurate, stable, and cost-effective means for biomarker detection, enhancing the diagnostic power of calprotectin. Further tests are underway to translate this breakthrough into a practical diagnostic product.
Common inflammatory disorders such as ulcerative colitis and Crohn’s disease can be diagnosed or monitored by measuring the protein calprotectin in stool samples, while serum levels of calprotectin could be used to monitor the inflammation status in rheumatoid arthritis. Calprotectin concentrations in patient samples are typically determined using antibodies that bind and detect the protein, e.g. in lateral flow assays like the now all-too-familiar home COVID-19 test kits.
But there is a problem with antibody-based calprotectin assays: the results can vary depending on the type of antibody and assay used. This happens because antibodies may bind to different sites on the protein, or might not have a uniform composition. The antibodies can also become inactivated over time due to unfolding or precipitation.

A lateral flow assay for calprotectin. Credit: Christian Heinis (EPFL)/Joëlle Jourdan (BÜHLMANN AG)
One possible solution is to use peptides instead of antibodies to detect and measure disease markers like calprotectin. Peptides are sequences of up to 50 amino acids that can bind to proteins with high affinity and selectivity, but, unlike antibodies, they can be chemically produced with high purity and homogeneity. In addition, peptides are stable over time, are cheaper to produce than antibodies and with lower inter-batch variability, and they can be attached to a specific location on a surface, significantly simplifying diagnostic assay development because it allows for a more accurate and controlled way of detecting biomarkers.
With this idea, Christian Gerhold, CTO of the diagnostics company BÜHLMANN, worked with the group of Professor Christian Heinis at EPFL to develop human calprotectin ligands based on peptides. From a library of more than 500 billion different peptides, Cristina Diaz-Perlas, a postdoc in Heinis’s group, isolated several binders of calprotectin, and showed that the peptides are suited for calprotectin quantification in simplified lateral flow assays. The best peptide had a dissociation constant of 26 nM – a measure of how tightly it binds calprotectin, making it a good candidate for diagnostic tests.
The peptide not only binds to a large surface region of calprotectin but also to a specific form of calprotectin that is the relevant species in patient samples. Under the guidance of Benjamin Ricken at BÜHLMANN, the peptide was finally tested in professionally assembled lateral flow cassettes and found that it was suited for accurate detection and quantification of calprotectin. In a proof-of-concept study, this setup was used to quantify the concentration of calprotectin in serum obtained from patient blood samples.
The peptide developed is the first synthetic affinity reagent that could be generated against the biomarker calprotectin. “The EPFL and BÜHLMANN teams are currently performing more tests with the calprotectin-specific peptide to translate the assay into a product that can bring the diagnostic power of this increasingly important biomarker to a new level to help patients suffering from inflammatory diseases,” says Christian Heinis.
Christian Gerhold adds: “This collaboration greatly benefited from BÜHLMANN’s knowhow to produce and handle the biomarker, and expertise of the EPFL team to generate and screen large combinatorial libraries of peptides by phage display.”
Reference: “High-affinity peptides developed against calprotectin and their application as synthetic ligands in diagnostic assays” by Cristina Díaz-Perlas, Benjamin Ricken, Lluc Farrera-Soler, Dmitrii Guschin, Florence Pojer, Kelvin Lau, Christian-Benedikt Gerhold and Christian Heinis, 17 May 2023, Nature Communications.
DOI: 10.1038/s41467-023-38075-7
Funding: Innosuisse – Schweizerische Agentur für Innovationsförderung


Be the first to comment on "Synthetic Peptides: A Game Changer for Inflammatory Disease Detection"