
Scientists have now proven that graphene is naturally permeable to protons. Using a technique called scanning electrochemical cell microscopy, they observed that protons not only move through the graphene crystal but also accelerate around its nanoscale wrinkles. This discovery, which defies previous theories, holds significant potential for advancing the hydrogen economy by replacing costly and environmentally harmful catalysts and membranes with sustainable 2D crystals.
Researchers have discovered that graphene naturally allows proton transport, especially around its nanoscale wrinkles. This finding could revolutionize the hydrogen economy by offering sustainable alternatives to existing catalysts and membranes.
Scientists from the University of Warwick and the University of Manchester have finally solved the long-standing puzzle of why graphene is so much more permeable to protons than expected by theory.
The saga began a decade ago, when scientists at The University of Manchester demonstrated that graphene is permeable to protons, nuclei of hydrogen atoms.
This finding was unexpected and contradicted theoretical predictions which suggested that it would take billions of years for a proton to pass through graphene’s dense crystalline structure. Due to this disparity, there was a theory suggesting that protons might be permeating through tiny holes, or pinholes, in the graphene structure rather than the crystal lattice itself.
In a recent publication in the journal Nature, a joint effort between the University of Warwick, spearheaded by Prof. Patrick Unwin, and The University of Manchester, led by Dr. Marcelo Lozada-Hidalgo and Prof. Andre Geim, presented their findings on this matter. Using ultra-high spatial resolution measurements, they conclusively demonstrated that perfect graphene crystals indeed allow proton transport. In a surprising twist, they also found that protons are strongly accelerated around nanoscale wrinkles and ripples present in the graphene crystal.
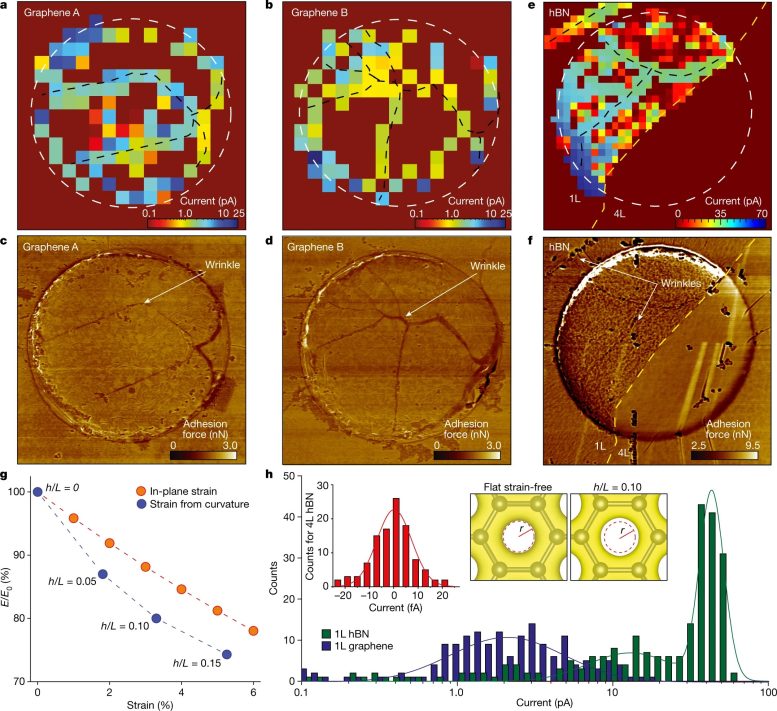
Unexpected inhomogeneity of proton transport through 2D crystals. Credit: Nature / DOI: 10.1038/s41586-023-06247-6
Implications for the Hydrogen Economy
This groundbreaking revelation carries immense significance for the hydrogen economy. The current mechanisms for generating and using hydrogen often rely on costly catalysts and membranes, some of which have notable environmental impacts. Replacing these with sustainable 2D crystals like graphene could play a pivotal role in advancing green hydrogen production, subsequently reducing carbon emissions and aiding the shift towards a Net Zero carbon environment.
To arrive at their conclusions, the researchers employed scanning electrochemical cell microscopy (SECCM). This technique allowed them to measure tiny proton currents in nanometer-sized regions, allowing the researchers to visualize the spatial distribution of proton currents through graphene membranes.
Had the proton movement been restricted to holes in the graphene, the currents would have been isolated to specific spots. However, no such concentrated currents were observed, debunking the theory about holes in the graphene structures.
What Is Graphene?
Graphene is a single layer of carbon atoms arranged in a 2D honeycomb lattice. It is renowned for its remarkable strength, conductivity, and thinness, making it one of the most promising and versatile materials in the fields of science and technology.
Researchers’ Comments and Observations
Dr. Segun Wahab and Dr. Enrico Daviddi, the lead authors of the study, expressed their astonishment at the absence of defects in the graphene crystals, stating, “We were surprised to see absolutely no defects in the graphene crystals. Our results provide microscopic proof that graphene is intrinsically permeable to protons.”
Unexpectedly, the proton currents were found to be accelerated around nanometer-sized wrinkles in the crystals. The scientists found that this arises because the wrinkles effectively ‘stretch’ the graphene lattice, thus providing a larger space for protons to permeate through the pristine crystal lattice. This observation now reconciles the experiment and theory.
Dr. Lozada-Hidalgo said: “We are effectively stretching an atomic scale mesh and observing a higher current through the stretched interatomic spaces in this mesh – this is truly mind-boggling.”
Prof. Unwin commented: “These results showcase SECCM, developed in our lab, as a powerful technique to obtain microscopic insights into electrochemical interfaces, which opens up exciting possibilities for the design of next-generation membranes and separators involving protons.”
The team is optimistic about how this discovery can pave the way for novel hydrogen technologies.
Dr. Lozada-Hidalgo said, “Exploiting the catalytic activity of ripples and wrinkles in 2D crystals is a fundamentally new way to accelerate ion transport and chemical reactions. This could lead to the development of low-cost catalysts for hydrogen-related technologies.”
Reference: “Proton transport through nanoscale corrugations in two-dimensional crystals” by O. J. Wahab, E. Daviddi, B. Xin, P. Z. Sun, E. Griffin, A. W. Colburn, D. Barry, M. Yagmurcukardes, F. M. Peeters, A. K. Geim, M. Lozada-Hidalgo and P. R. Unwin, 23 August 2023, Nature.
DOI: 10.1038/s41586-023-06247-6








“Using ultra-high spatial resolution measurements, they conclusively demonstrated that perfect graphene crystals indeed allow proton transport. In a surprising twist, they also found that protons are strongly accelerated around nanoscale wrinkles and ripples present in the graphene crystal.
This groundbreaking revelation carries immense significance for the hydrogen economy. The current mechanisms for generating and using hydrogen often rely on costly catalysts and membranes.”
Fantastic work. Thank you.
It means water should must be dessociated before using graphene.
Hats off to the amazing scientists and researchers everywhere. You guys are the great hope for our planet. Keep up the good work!
One thing never mentioned about the “hydrogen economy” is that burning hydrogen doesn’t just produce water but also sulfuric acid from the sulfur dioxide in the air. Now on a small scale this means nothing but on a huge scale it means a lot. Why this is never mentioned or ignored makes me wonder……
I think Fritz left a very good, that should be addressed
How does that work, Fritz? There’s no sulfate in the equation.