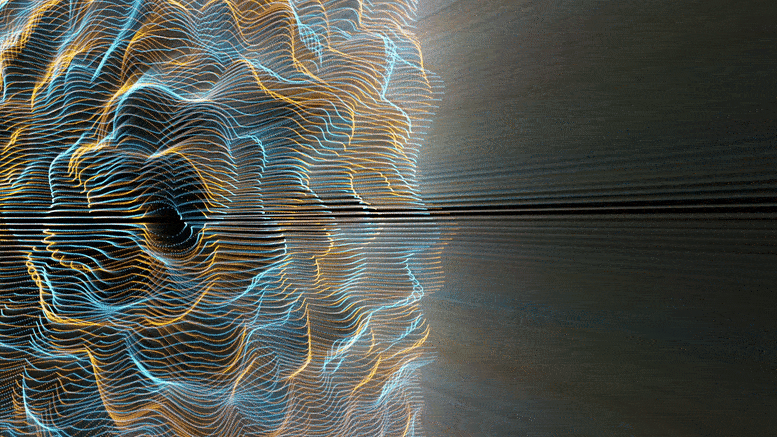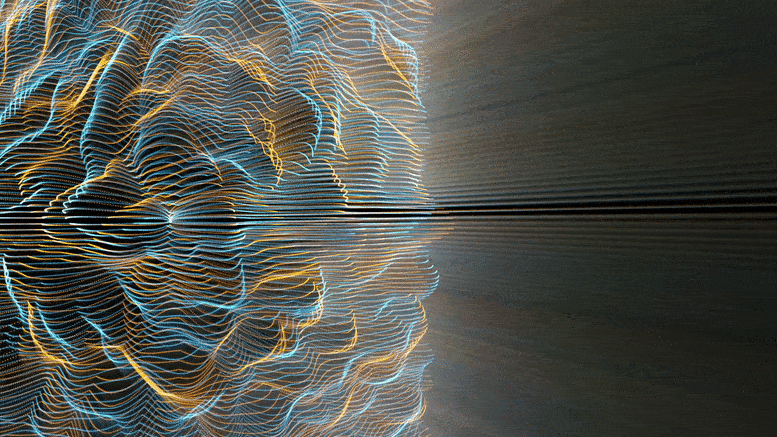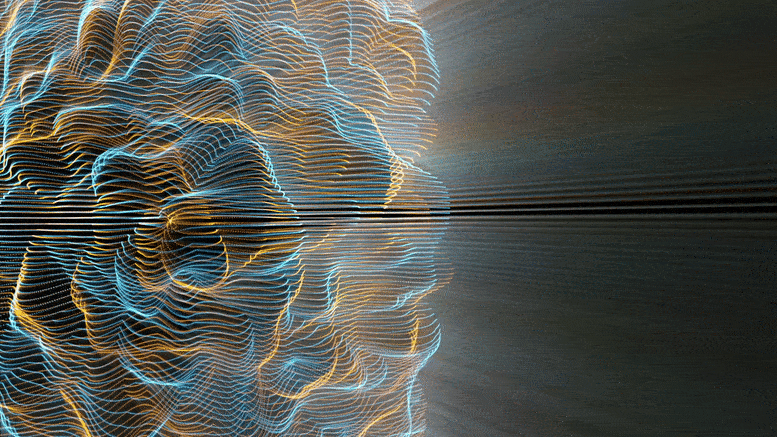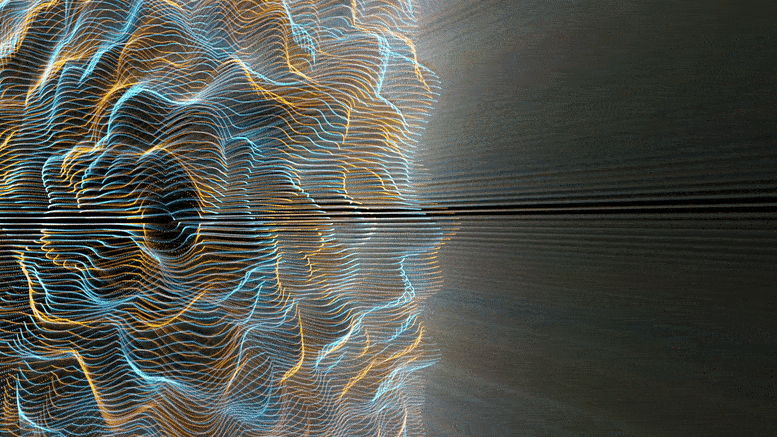
Chemical reactions were found to accelerate Brownian diffusion by generating long-range ripples in the surrounding solvent, defying the conventional notion that diffusion and reactions are unrelated. This discovery highlights the natural self-stirring effect of chemical reactions.
IBS researchers in South Korea extend the understanding of energy flow in chemical reactions and show that it may produce useful molecular swimmers.
Steve Granick, Director of the IBS Center for Soft and Living Matter, and Dr. Huan Wang, Senior Research Fellow, report together with 5 interdisciplinary colleagues in the July 31 issue of the journal Science that common chemical reactions accelerate Brownian diffusion by sending long-range ripples into the surrounding solvent.
The findings violate a central dogma of chemistry, that molecular diffusion and chemical reaction are unrelated. To observe that molecules are energized by chemical reaction is “new and unknown,” said Granick. “When one substance transforms to another by breaking and forming bonds, this actually makes the molecules move more rapidly. It’s as if the chemical reactions stir themselves naturally.”
“Currently, Nature does an excellent job of producing molecular machines but in the natural world scientists have not understood well enough how to design this property,” said Wang. “Beyond curiosity to understand the world, we hope that practically this can become useful in guiding thinking about transducing chemical energy for molecular motion in liquids, for nanorobotics, precision medicine, and greener material synthesis.”
The unexpected ripples generated by chemical reactions, especially when catalyzed (accelerated by substances not themselves consumed), propagate long-range. For chemists and physicists, this work challenges the textbook view that molecular motion and chemical reaction are decoupled, and that reactions affect only the nearby vicinity. For engineers, this work shows a powerful new approach to design nanomotors at the truly molecular level.
Screening 15 organic chemical reactions, the researchers study chemical reactions that are workhorses with wide application within the organic chemical, pharmaceutical and materials industries. For example, “click” reactions assist the assembly of libraries of biomedical compounds for screening, and the “Grubbs” reaction used for plastic manufacture. Their economic impact is major. Estimates indicate that a majority of all products manufactured require catalysis somewhere in their production sequence.
Wang remarked with enthusiasm: “Now, we’re like a baby taking her first steps and there’s so much exciting opportunity to grow this baby.”
In designing their study, the researchers were bio-inspired by noticing that motion can be powered by enzymes and other molecular motors that are prevalent in living systems. Pioneering earlier work by Dr. Ah-Young Jee in the same research center showed this. But there was no consensus among scientists if these reports could be correctly extended outside biology. Analyzing the problem, the researchers made a high-risk, high-payoff argument. They hypothesized that the phenomenon would form an approach to understand molecular machines in the real world.
Testing their hypothesis, the team developed new analytical techniques. Professor Tsvi Tlusty, a theorist, predicted that catalysts in reaction gradients should migrate “uphill” in the direction of lesser diffusivity. Professor Yoon-Kyoung Cho, a microfluidics expert, designed a tailor-made microfluidics chip to test this idea. Dr. Ruoyu Dong, a Research Fellow, performed numerical computer simulations. “Our interdisciplinary team responded incredibly quickly to the research opportunities thanks to the research freedom of the Korean Institute for Basic Science,” said Granick.
The team presents guidelines showing that the magnitude of diffusion increase in different systems depends on the energy release rate. These guidelines can be useful practically to estimate the effect in as-yet untested reactions. Beyond this, the study is very useful for expanding understanding of active materials, a collective term that traditionally refers to things like cells and microorganisms.
Granick concluded: “The field of active materials, quite new and growing fast, is enriched by this discovery that chemical reactions behave as nanoswimmers made of individual molecules that stir up the reaction soup. The concept of active materials has shown its value in challenging a central dogma of chemistry.”
These findings were published in the July 31, 2020 issue of Science magazine. The study was performed at the IBS Center for Soft and Living Matter by authors Huan Wang, Myeonggon Park, Ruoyu Dong, Junyoung Kim, Yoon-Kyoung Cho, Tsvi Tlusty, and Steve Granick.
Reference: “Boosted molecular mobility during common chemical reactions” by Huan Wang, Myeonggon Park, Ruoyu Dong, Junyoung Kim, Yoon-Kyoung Cho, Tsvi Tlusty and Steve Granick, 31 July 2020, Science.
DOI: 10.1126/science.aba8425






Be the first to comment on "Unexpected Ripples Generated by Chemical Reactions Violate a Central Tenet of Chemistry"