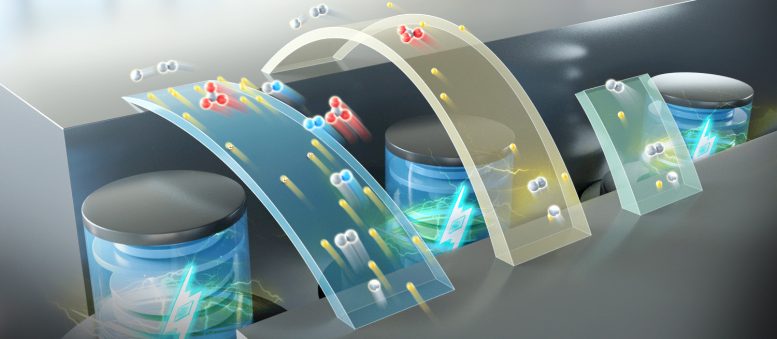
Researchers at the Dalian Institute of Chemical Physics have developed an innovative aqueous battery that reaches a specific capacity of 840 Ah/L and an energy density of 1200 Wh/L, enhancing both safety and energy efficiency in battery technology. Credit: DICP
Traditional lithium-ion batteries, while offering high energy density, have compromised safety because they use flammable organic electrolytes.
Aqueous batteries use water as the solvent for electrolytes, significantly enhancing the safety of the batteries. However, due to the limited solubility of the electrolyte and low battery voltage, aqueous batteries typically have a lower energy density. This means that the amount of electricity stored per unit volume of aqueous battery is relatively low.
In a new study published in Nature Energy, a research group led by Prof. Li Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Fu Qiang’s group also from DICP, developed a multi-electron transfer cathode based on bromine and iodine, realizing a specific capacity of more than 840 Ah/L, and achieving an energy density of up to 1200 Wh/L based on catholyte in full battery testing.
Technical Enhancements and Achievements
To improve the energy density of aqueous batteries, researchers used a mixed halogen solution of iodide ions (I–) and bromide ions (Br–) as the electrolyte. They developed a multi-electron transfer reaction, transferring I– to iodine element (I2) and then to iodate (IO3–). During the charging process, I– was oxidized to IO3– on the positive side, and the generated H+ was conducted to the negative side in the form of a supporting electrolyte. During the discharge process, H+ was conducted from the positive side, and IO3– was reduced to I–.
The developed multi-electron transfer cathode had a specific capacity of 840 Ah/L. Combining the cathode with metallic Cd to form a full battery, researchers achieved an energy density up to 1200 Wh/L based on the developed catholyte.
Besides, researchers confirmed that Br– added to the electrolyte could generate polar iodine bromide (IBr) during the charging process, which facilitated the reaction with H2O to form IO3–. During the discharge, IO3– could oxidize Br– to Br2 and participated in the electrochemical reaction to realize reversible and rapid discharge of IO3–. Therefore, the bromide intermediate formed during the charge and discharge process optimized the reaction process, effectively improving the kinetic and reversibility of the electrochemical reaction.
Prof. Fu’s group proved the multi-electron transfer process through in-situ optical microscopy, Raman spectroscopy and so on.
“This study provides a new idea for the design of high-energy-density aqueous batteries, and may expand the aqueous batteries application in power batteries field,” said Prof. Li.
Reference: “Reversible multielectron transfer I−/IO3− cathode enabled by a hetero-halogen electrolyte for high-energy-density aqueous batteries” by Congxin Xie, Chao Wang, Yue Xu, Tianyu Li, Qiang Fu and Xianfeng Li, 23 April 2024, Nature Energy.
DOI: 10.1038/s41560-024-01515-9








Be the first to comment on "Unlocking High Energy: New Aqueous Batteries Surpass Traditional Limits"