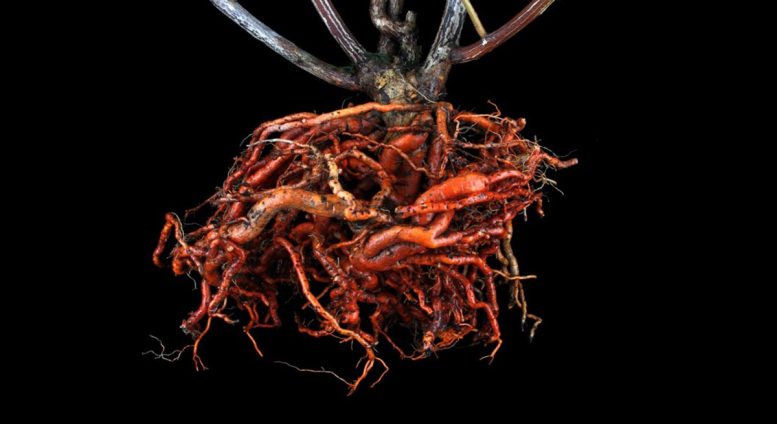
Root of Tripterygium wilfordii Celastrol is produced in the root of the traditional medicinal plant Tripterygium wilfordii. Credit: Nikolaj Hansen
A traditional Chinese medicinal plant has huge potential as a weight loss drug. However, due to the plant’s notorious toxicity, no one has yet succeeded in exploiting it effectively. Researchers from the University of Copenhagen have not only found the formula for the synthetic production of the compound – they have even found a simple and sustainable recipe using ordinary yeast as the main ingredient.
In China, children are taught to steer clear of this plant. The plant, Thunder god vine (Chinese: 雷公藤), which in China has earned the nickname “Seven Steps to Death,” is so poisonous that a person risks death only a few steps after consuming it.
But despite its deadliness, the Thunder god vine (Tripterygium wilfordii) hides something quite beneficial to us humans as well. Within its roots, the plant produces the compound celastrol, a chemical agent with powerful anti-obesity properties.
Experiments using mice on a high fat diet have shown that the mice given celastrol gained 45% less weight than the control group. Experiments with human cells have shown similar effects.
HOW THE DRUG WORKS
Some people are able to eat a lot without gaining weight because our metabolism knows when we eat a lot and secretes certain hormones that cause us to burn more calories. One of these hormones is leptin.
People who are overweight become resistant to leptin due to an overproduction of the hormone, which leads to an inflammation of the hypothalamus. As a result, the body does not receive the signal. Celastrol re-sensitizes the body to leptin.
The effect is due to the fact that celastrol reactivates the body’s sensitivity to leptin, a hormone to which overweight people become resistant. Leptin is one of the hormones that causes the body to burn more calories and thereby regulate weight.
How does one get their hands on the ‘good’ substance without the toxicity that typically accompanies it? That is the question.
“For obvious reasons, a person cannot just eat the plant and benefit from the drug. So what do we do? The problem with extracting celastrol from the nature source is that it is very hard to separate it from the other toxic molecules that the plant is full of. So far, there has been no effective method to achieve this,” says Sotirios Kampranis, professor at the Department of Plant and Environmental Sciences.

Yong Zhao and Sotirios Kampranis
Yong Zhao and Sotirios Kampranis with celastrol produced in yeast cells Credit: Yao-Tao Duan
Yeast as ‘surrogate mother’
In approaching the problem, the research team from the Department of Plant and Environmental Sciences sought to find a way to produce the substance biotechnologically. Led by Assistant Professor Yong Zhao, the researchers were the first to map the pathway with all 15 biochemical steps that the plant undergoes as it forms celastrol. These steps are vital to know in order to recreate the substance biosynthetically.
THUNDER GOD VINE
The plant, Thunder god vine, which in Latin is called Tripterygium wilfordii, has been used for thousands of years in traditional Chinese medicine to treat rheumatoid arthritis and as a contraceptive.
The plant grows in the mountainous regions of Southern China, where local collectors harvest it. Thus, in addition to being extremely toxic, it is also difficult to find.
“The compound is extracted from the plant root. And the plant takes at least three years to grow large enough for it to be harvested. Therefore, an entire plant must be sacrificed to acquire the drug. So it is not a tenable way,” Sotirios Kampranis points out.
“We found out how the plant forms celastrol by finding all of the steps in the manufacturing process. This means that we could take the genes and enzymes that make the substance and put them into another organism that does not produce toxic substances. And that’s what we’ve done with yeast,” says Yong Zhao.
The researchers were able to produce synthetic celastrol in a tank with ordinary yeast that was used as a host organism within which the substance was produced.
“Imagine, you just need to feed the celastrol molecule table sugar, and by doing so, you get a compound that is almost in pure form without the toxic compounds that otherwise come with it in nature. The process is simple and effective – it only takes around a week to get the final product. And it takes place without the toxic solvents or catalysts that are typically used in chemical syntheses. I believe there is a huge potential here,” says Sotirios Kampranis.

Isolated celastrol
Isolated celastrol produced by the Kampranis lab. Celastrol is a complex plant specialized metabolite with a reddish color. Credit: Yong Zhao
The greenest – and only – method
Today, most pharmaceuticals are created through synthesis based on crude oil-based petrochemicals. And the traditional ways of developing synthetic drugs are actually not even an option, as Yong Zhao explains:
“Because the celastrol molecule is so complex, there are currently only very inefficient chemical synthesis methods which are not applicable for large-scale production. So our method is not only a green method – it is also the only real method that exists.”
The researchers point out that yeast is widely used in the biotech industry, where one finds all the necessary know-how and infrastructure to produce celastrol at a large scale.
“That is precisely why we have chosen to use yeast as an organism. While academics develop the technology, it is important that it comes in a form that is useful for industry, where the technology can be further developed to make products that can help us all,” says Sotirios Kampranis.
The next step is more closely investigating the drug’s potential to treat obesity in humans. The researchers hypothesize that a potential treatment can either be done with celastrol alone or combined with other therapies.
“For example, one can imagine a treatment where celastrol is combined with other anti-obesity drugs to achieve a more robust effect. Because the more targets in the body that are hit, the better. Indeed, one often sees a synergistic effect when several agents are at play simultaneously. But here, of course, the pharmaceutical industry must take over,” says Yong Zhao.
Reference: “Biosynthesis and biotechnological production of the anti-obesity agent celastrol” by Yong Zhao, Nikolaj L. Hansen, Yao-Tao Duan, Meera Prasad, Mohammed S. Motawia, Birger L. Møller, Irini Pateraki, Dan Staerk, Søren Bak, Karel Miettinen and Sotirios C. Kampranis, 26 June 2023, Nature Chemistry.
DOI: 10.1038/s41557-023-01245-7
The researchers behind the study are: Yong Zhao, Nikolaj L. Hansen, Yao-Tao Duan, Meera Prasad, Mohammed S. Motawia, Birger L. Møller, Irini Pateraki, Dan Staerk, Søren Bak, Karel Miettinen and Sotirios C. Kampranis, all from the Department of Plant and Environmental Sciences and the Department of Drug Design and Pharmacology at the University of Copenhagen.
The University of Copenhagen has filed for a patent application for the invention and is currently in talks with potential partners about commercializing the method.
The project is supported by the Novo Nordisk Foundation.








Be the first to comment on "Weight Loss Drug From Highly Toxic Plant Can Now Be Produced Simply and Sustainably"