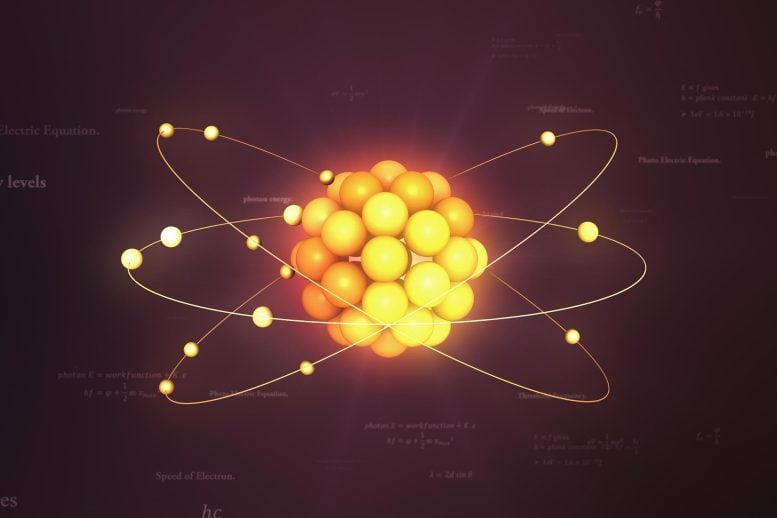
Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. Electrons can absorb energy and escape from the nucleus, forming ions when they lose or gain electrons.
What Are Electrons?
The electron is a subatomic particle that is present in all atoms. Unlike protons, neutrons, or the nuclei of atoms, electrons are elementary particles. This means they are not composed of even smaller particles. Also unlike protons and neutrons, electrons have essentially no mass. Finally, electrons are different from protons and neutrons in that they surround the nucleus instead of being part of the nucleus.
Normally, electrons are bound to the nuclei of atoms. This happens because electrons have a negative charge that interacts with the positive charge of the nucleus of an atom. In a neutral atom, the number of electrons is the same as the number of positive charges in the nucleus.
All the elements of the Periodic Table are neutral. However, any atom can have more or fewer electrons than positive charges. This makes the atom negatively or positively charged. These charged atoms are known as ions. More specifically, negatively charged ions are called anions and positively charged ions are called cations.
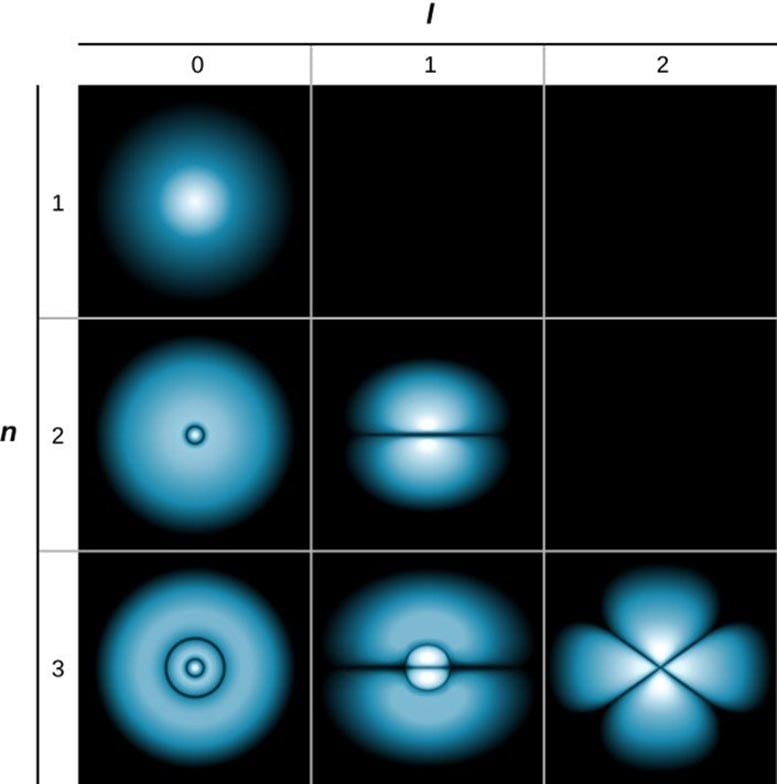
This image shows the probability of finding hydrogen’s electron in the base (n=1) and excited states (n=2 and n=3). Light areas indicate higher probability. The rows (l) indicate the electron’s angular momentum. Credit: University Physics Vol 3 by OpenStax (CC BY 4.0)
Atoms lose electrons because of how they interact with forces beyond atomic nuclei. When an electron gains extra energy, it can become excited. This can happen when an electron absorbs a photon (a packet of light) or collides with a nearby atom or particle. Electrons can gain enough energy to leave atomic nuclei behind. These free electrons mix with ions to form a plasma.
Electrons can separate from atoms because of the way they surround nuclei. In the past, scientists believed electrons orbited nuclei in the same way the moon orbits the Earth. However, scientists now believe electrons surround nuclei in a cloud divided into shells. These shells are similar to the different layers in the Earth’s atmosphere. However, electrons do not exist in specific points in these shells. Because of quantum mechanics, electrons act as both points and waves. This means the areas within the shells have different probabilities of containing electrons.
Atoms have multiple shells, each with a different number of subshells. Shells can hold more and more electrons the further they are from the nucleus, and electrons have higher and higher energy levels as they move to shells away from the nucleus.
If the two outer shells are full, the element is much less reactive with other elements. This low reactivity is why the elements in the last column of the periodic table—helium, neon, etc.—are called the “noble gases” because they don’t react.
When electrons become “excited” and gain energy, then “relax” and return to their original shells, the atom emits a photon. The wavelength of the photon depends on how far the electron falls back toward the atom’s nucleus. These wavelengths are specific to each element.
Fast Facts
- Use this interactive periodic table to learn about electrons and other components of the elements
- Electrons can “tunnel” and appear on the opposite side of a barrier. It’s a phenomenon called quantum tunneling, and it’s possible because electrons are both particles and waves, and part of a wave can be on the other side of a barrier. This phenomenon is used in electron microscopes and modern integrated circuits.
DOE Office of Science: Contributions to Subatomic Particle Research
The DOE Office of Nuclear Physics in the Office of Science supports research to understand all forms of nuclear matter and the subatomic particles that make up atomic nuclei. This research includes unraveling previously unknown properties of atoms and the subatomic particles they are composed of in their natural state for important applications in medicine, commerce, and national defense. Another area of study is understanding precisely how nuclei are structured depending on the number of protons and neutrons inside them. Other research focuses on heating nuclei to the temperature of the early universe to understand how they condensed out of the quark-gluon soup that existed at the time.







I had always thought of the electron orbiting the nuclei to shape a cocoon around the center element separating each different partnering element. the direction of the electron path shaping the cocoon at each different pass would be the spin.To create the larger elements parts per trillion or billion would match the spin and if compatible will merge for a heavier element, a lot of other cooking has to happen at the same time, charge state, thermal dynamics,pressure.
This is backwards:
More specifically, negatively charged ions are called anions and positively charged ions are called cations.