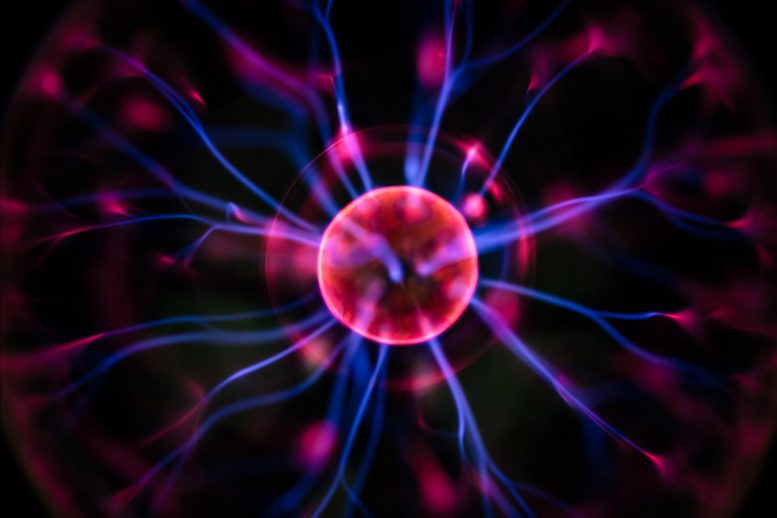
Plasma, making up 99% of the visible universe, exists predominantly in stars and space. It forms when electrons detach from atoms, creating ions and free electrons, a state that can conduct electricity and exhibits complex behaviors.
What Is Plasma?
Plasma is one of the four fundamental states of matter, alongside gases, liquids, and solids. While most people don’t think about plasma in their daily lives the way they think about other states of matter, plasma constitutes 99% of the visible matter in the universe. This includes astrophysical plasma found in outer space, such as within nebulae and stars like the Sun.
In plasma, some of the electrons separate and become free from neutral atoms (atoms that have an equal number of protons and electrons and thus a neutral charge). The resulting free electrons make plasma different from the other states of matter, where the electrons remain closely held to nuclei.
When the atoms in plasma separate from their negatively charged electrons, they no longer have a neutral electrical charge. Instead, the atoms become ions—positively charged particles. Therefore, plasma is an ionized state made up of positively charged ions and negatively charged electrons.

The aurora borealis forms from particles colliding in waves in plasma in the Earth’s atmosphere. Credit: Frank Olsen
There are several reasons why electrons in atoms can separate and form plasma. In laboratory experiments, scientists can blast the atoms with high-voltage electricity, lasers, or electromagnetic fields to form plasma. In space, plasma may form from high-energy photons including gamma rays striking atoms. Plasma can also form in space when gravity increases the pressure so much that it superheats gases. The high temperatures cause atoms to collide with each other, resulting in the electrons separating from the atoms, creating plasma and the beginnings of a star.
This process of gases being superheated to create plasma suggests that gases and plasma have a relationship similar to how liquid can be a heated form of a solid. This analogy is not always correct. For one, unlike gas, plasma can conduct electricity. Also, in gases, all the particles behave in similar ways. In plasma, however, electrons and ions behave and interact in very complex ways, which creates waves and instabilities.
Plasmas come in several types. Most plasma in the universe is what researchers call high-temperature plasmas. In these high-temperature plasmas, temperatures can be more than 10,000 degrees Fahrenheit, and all the atoms can be fully ionized. Low-temperature plasmas are different. The atoms are only partially ionized, and they can be incredibly cool—even room temperature. Another unusual type of plasma is high-energy density plasma, which scientists create in laboratories to study their unusual properties.
Fast Facts
- One type of lightning – ball lightning – is plasma. Learn more from the Max Planck Institute.
- The aurora borealis is also caused by plasma. Learn more in this science highlight.
- Confining plasma is an important step in designing fusion tokamak and stellarator devices that may eventually provide us with fusion power.
- High-energy density plasma science enabled fusion ignition in laboratory conditions.
DOE Office of Science: Contributions to Plasma Research
Studying plasma helps scientists understand matter. It also helps them progress toward the goal of fusion energy. The Department of Energy (DOE) Office of Science supports research into plasma through its Fusion Energy Sciences and Nuclear Physics programs. DOE-funded research on plasma has also improved the manufacturing of the semiconductors found in everything from phones and computers to cars. Expertise in plasma helped researchers at the DOE National Laboratories develop ways to control the creation of semiconductors on an atom-by-atom level.








This is informative, thanks for sharing.