Once thought unlikely, this new finding in coordination chemistry could lead to promising advances in catalysis and materials science.
For more than 100 years, the widely accepted 18-electron rule has been a foundational guideline in organometallic chemistry. Now, researchers at the Okinawa Institute of Science and Technology (OIST) have synthesized a new organometallic compound that challenges this principle. They developed a stable 20-electron version of ferrocene, an iron-based metal-organic complex, which could open new directions in chemical research.
“For many transition metal complexes, they are most stable when surrounded by 18 formal valence electrons. This is a chemical rule of thumb on which many key discoveries in catalysis and materials science are based,” said Dr. Satoshi Takebayashi, lead author of the paper published in Nature Communications, working with colleagues from Germany, Russia, and Japan. Ferrocene is a classic example that follows this rule. “We have now shown for the first time that it is possible to synthesize a stable 20-electron ferrocene derivative,” he added.
This achievement advances our understanding of metallocenes, a class of compounds with a distinctive “sandwich” structure where a metal atom is positioned between two organic rings.
Rebuilding our conceptual understanding
First created in 1951, ferrocene transformed the field of chemistry with its surprising stability and distinctive structure. Its discovery led to the 1973 Nobel Prize in Chemistry and marked a turning point in the understanding of metal–organic bonding. Ferrocene became a cornerstone of modern organometallic chemistry and has continued to inspire scientists to investigate the potential of metal–organic compounds.
Building on this legacy, the new study introduces a significant advancement. The research team developed a custom ligand system that successfully stabilized a ferrocene derivative containing 20 valence electrons—an arrangement once thought unlikely in coordination chemistry. “Moreover, the additional two valence electrons induced an unconventional redox property that holds potential for future applications,” said Dr. Takebayashi.
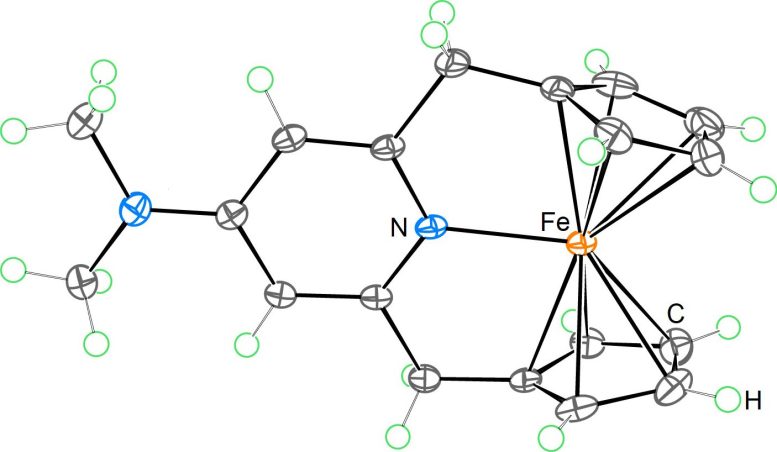
This finding is notable because, although ferrocene is commonly used in redox reactions (processes involving electron transfer), it has traditionally been restricted to a narrow set of oxidation states. By forming an Fe–N bond in this new compound, the researchers expanded ferrocene’s ability to gain or lose electrons, opening up new possibilities for its use in catalysis and functional materials. Applications could range from energy storage systems to chemical manufacturing.
Learning how to push beyond the traditional rules of chemical stability allows scientists to design molecules with specific, desirable properties. These discoveries may pave the way for progress in sustainable chemistry, including the creation of environmentally friendly catalysts and advanced new materials.
A platform for future innovation
Ferrocene derivatives have already made their way into various technologies, from solar cells and pharmaceuticals to medical devices and advanced catalysts. By expanding the conceptual toolkit available to chemists, this latest breakthrough could help build on and diversify these applications while inspiring entirely new ones.
The Organometallic Chemistry Group at OIST focuses on uncovering the fundamental principles that govern metal-organic interactions and applying them to real-world challenges. The team has a special interest in unconventional compounds that defy standard chemical rules, such as the 20-electron ferrocene derivative reported in this study.
Reference: “From 18- to 20-electron ferrocene derivatives via ligand coordination” by Satoshi Takebayashi, Jama Ariai, Sergey V. Kartashov, Robert R. Fayzullin, Tomoko Onoue, Ko Mibu, Hyung-Been Kang and Noriko Ishizu, 7 July 2025, Nature Communications.
DOI: 10.1038/s41467-025-61343-7
Never miss a breakthrough: Join the SciTechDaily newsletter.
1 Comment
It may challenge our knowledge of the principles, not the principles themselves.