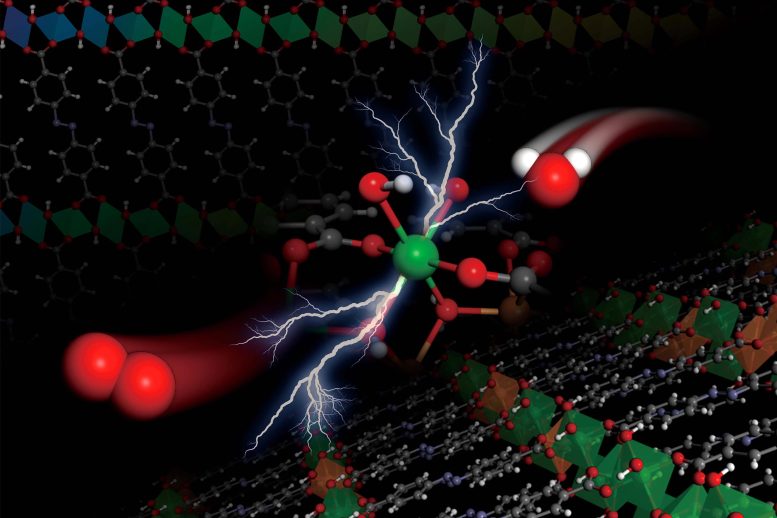
Illustration depicts an electrochemical reaction, splitting water molecules (at right, with oxygen atom in red, and two hydrogen atoms in white) into oxygen molecules (at left), taking place within the structure of the team’s metal hydroxide organic frameworks, depicted as the lattices at top and bottom. Credit: Courtesy of the researchers
The material could replace rare metals and lead to more economical production of carbon-neutral fuels.
An electrochemical mechanism that splits apart water molecules to produce oxygen is at the core of many approaches aimed at producing alternative transportation fuels. However, this reaction requires the use of a catalyst material, and current versions need the use of rare and costly materials such as iridium, limiting the potential of such fuel generation.
Researchers at MIT and elsewhere have created a completely new form of catalyst material known as a metal hydroxide-organic framework (MHOF), which is composed of affordable and abundant components. Due to the family of materials, engineers can carefully adapt the catalyst’s shape and composition to the demands of a specific chemical process. It can then equal or surpass the performance of conventional, more costly catalysts.
The findings are described on February 24, 2022, in the journal Nature Materials, in a paper by MIT postdoc Shuai Yuan, graduate student Jiayu Peng, Professor Yang Shao-Horn, Professor Yuriy Román-Leshkov, and nine others.
Oxygen evolution reactions are one of the reactions common to the electrochemical production of fuels, chemicals, and materials. These processes include the generation of hydrogen as a byproduct of the oxygen evolution, which can be used directly as a fuel or undergo chemical reactions to produce other transportation fuels; the manufacture of ammonia, for use as a fertilizer or chemical feedstock; and carbon dioxide reduction in order to control emissions.
But without help, “these reactions are sluggish,” Shao-Horn says. “For a reaction with slow kinetics, you have to sacrifice voltage or energy to promote the reaction rate.” Because of the extra energy input required, “the overall efficiency is low. So that’s why people use catalysts,” she says, as these materials naturally promote reactions by lowering energy input.
But until now, these catalysts “are all relying on expensive materials or late transition metals that are very scarce, for example, iridium oxide, and there has been a big effort in the community to find alternatives based on Earth-abundant materials that have the same performance in terms of activity and stability,” Román-Leshkov says. The team says they have found materials that provide exactly that combination of characteristics.
Other teams have explored the use of metal hydroxides, such as nickel-iron hydroxides, Román-Leshkov says. But such materials have been difficult to tailor to the requirements of specific applications. Now, though, “the reason our work is quite exciting and quite relevant is that we’ve found a way of tailoring the properties by nanostructuring these metal hydroxides in a unique way.”
The researchers drew inspiration from previous research on a comparable class of chemicals known as metal-organic frameworks (MOFs), which are a kind of crystalline structure composed of metal oxide nodes linked together by organic linker molecules. The researchers discovered that by substituting the metal oxide in such materials with particular metal hydroxides, it was possible to create precisely tunable materials with the necessary stability to be potentially useful as catalysts.
“You put these chains of these organic linkers next to each other, and they actually direct the formation of metal hydroxide sheets that are interconnected with these organic linkers, which are then stacked, and have a higher stability,” Román-Leshkov says. This has multiple benefits, he says, by allowing a precise control over the nanostructured patterning, allowing precise control of the electronic properties of the metal, and also providing greater stability, enabling them to stand up to long periods of use.
In testing such materials, the researchers found the catalysts’ performance to be “surprising,” Shao-Horn says. “It is comparable to that of the state-of-the-art oxide materials catalyzing for the oxygen evolution reaction.”
Being composed largely of nickel and iron, these materials should be at least 100 times cheaper than existing catalysts, they say, although the team has not yet done a full economic analysis.
This family of materials “really offers a new space to tune the active sites for catalyzing water splitting to produce hydrogen with reduced energy input,” Shao-Horn says, to meet the exact needs of any given chemical process where such catalysts are needed.
The materials can provide “five times greater tunability” than existing nickel-based catalysts, Peng says, simply by substituting different metals in place of nickel in the compound. “This would potentially offer many relevant avenues for future discoveries.” The materials can also be produced in extremely thin sheets, which could then be coated onto another material, further reducing the material costs of such systems.
So far, the materials have been tested in small-scale laboratory test devices, and the team is now addressing the issues of trying to scale up the process to commercially relevant scales, which could still take a few years. But the idea has great potential, Shao-Horn says, to help catalyze the production of clean, emissions-free hydrogen fuel, so that “we can bring down the cost of hydrogen from this process while not being constrained by the availability of precious metals. This is important, because we need hydrogen production technologies that can scale.”
Reference: “Tunable metal hydroxide–organic frameworks for catalysing oxygen evolution” by Shuai Yuan, Jiayu Peng, Bin Cai, Zhehao Huang, Angel T. Garcia-Esparza, Dimosthenis Sokaras, Yirui Zhang, Livia Giordano, Karthik Akkiraju, Yun Guang Zhu, René Hübner, Xiaodong Zou, Yuriy Román-Leshkov and Yang Shao-Horn, 24 February 2022, Nature Materials.
DOI: 10.1038/s41563-022-01199-0
The research team included others at MIT, Stockholm University in Sweden, SLAC National Accelerator Laboratory, and Institute of Ion Beam Physics and Materials Research in Dresden, Germany. The work was supported by the Toyota Research Institute.






Aluminum alloy was’nt mentioned in the article as a hydrogen liberation catalyst. Recommend stating the specific and volumetric energy desnity of the material to make an apples to apples comparison.
What do you think of HIPPER VOLTIC HHO as designed by Michael Archer (current) on Facebook