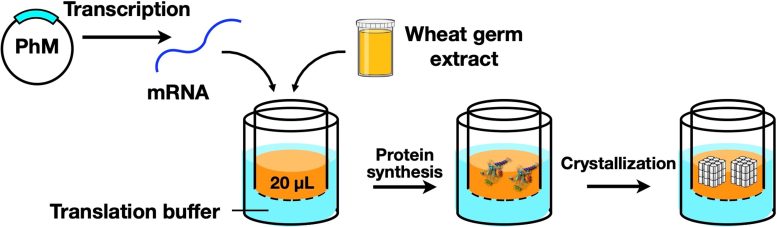
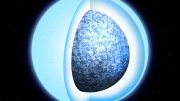
Schematic illustration of CFPC process using a wheat germ protein synthesis kit to synthesis polyhedrin monomer (PhM) which was further crystallized to nano-sized polyhedra crystals. Credit: Prof. Takafumi Ueno
Tokyo Tech developed a new cell-free protein crystallization (CFPC) method that includes direct protein crystallization and is a major advancement in the field of structural biology. This technique will enable the analysis of unstable proteins that couldn’t be studied using conventional methods. Analyzing these will increase our knowledge of cellular processes and functions.
Most of us are familiar with certain crystals like salt and sugar that we use in our everyday life. However, there is another set of crystals, hidden from the naked eye, that is crucial to our biology. In living cells, microscopic protein crystals help sustain processes like immune system activation, protein storage, and protection.
Scientists developed the in-cell protein crystallization (ICPC) method to better understand the relationship between protein crystals’ structure and function. This method can directly observe protein crystals in living cells, ensuring high-quality crystals without the need for purification processes or complex screening methods. However, despite its many advantages, very few structures were reported because the crystals formed in living cells didn’t have the size and quality that was required for analysis. So, a team of scientists from Japan, led by Prof. Takafumi Ueno of Tokyo Tech aimed to develop a better method. And recently, they hit a breakthrough!
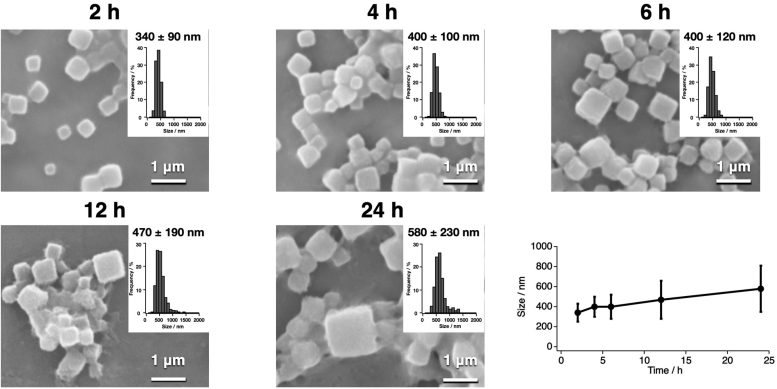
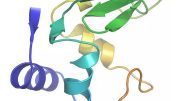
Scanning electron microscopy images and size distribution histograms of polyhedra crystals (PhCs) show various points in the time-dependent CFPC process. Credit: Prof. Takafumi Ueno
In their article, which will be published today (October 3) in Scientific Reports, the team reported the development of a technique that would make protein crystallization and analysis more efficient and effective. This technique—a cell-free protein crystallization (CFPC) method—was a hybrid between in vitro protein crystallization and ICPC, and allowed the rapid and direct formation of protein crystals without the need for complicated crystallization and purification methods.
“ICPC is expected to become an important tool in crystal structure analysis but we need a method to obtain better resolution protein crystal structures. So, we focused on establishing high-quality protein crystallization using CFPC with small-scale and rapid reactions,” says Prof. Ueno, who also heads the Ueno Laboratory at Tokyo Tech. Members of this lab study naturally occurring protein assemblies, their structure, and their functions, aiming to apply this knowledge to develop innovative biotechnology and energy solutions. (Some of the scientists on the research team conducting the current study are also members of the Ueno Laboratory.)
The research team used a wheat germ protein synthesis kit, which is a tool for the synthesis of polyhedrin monomer, a viral protein produced in insect cells by cypovirus infection. This protein was then crystallized using the new CFPC method, leading to the formation of nano-sized polyhedra crystals (PhCs). The scientists could efficiently complete this process within 6 hours, using only 20 microlitres of the reaction mixture. Scanning electron microscopy images indicated that the PhCs had excellent purity, which allowed the determination of their structure at a resolution as high as 1.95 Å (or 1.95 angstrom). To further explore the capabilities of their new system, the researchers carried out the structural analysis of crystalline inclusion protein A (CipA). Its structure was determined at a high resolution of 2.11 Å, something that had never been reported before this study.
This work is a major leap forward in the field of structural biology as the method it proposes will enable the analysis of unstable and low-yield proteins that cannot be studied via conventional methods. This technology also aims to aid in the development of advanced techniques for small-scale and rapid protein crystallization and analysis. “The high-quality protein crystals produced by our method will expand the horizons of structural determination and provide us with useful and unprecedented insights into the complex environment of living cells” concludes Prof Ueno.
A crystal-clear view of the crystalline proteins indeed!
Reference: “Cell-free Protein Crystallization for Nanocrystal Structure Determination” 3 October 2022, Scientific Reports.
DOI: 10.1038/s41598-022-19681-9




Be the first to comment on "Advancing Structural Biology With Novel Cell-Free Protein Crystallization Method"