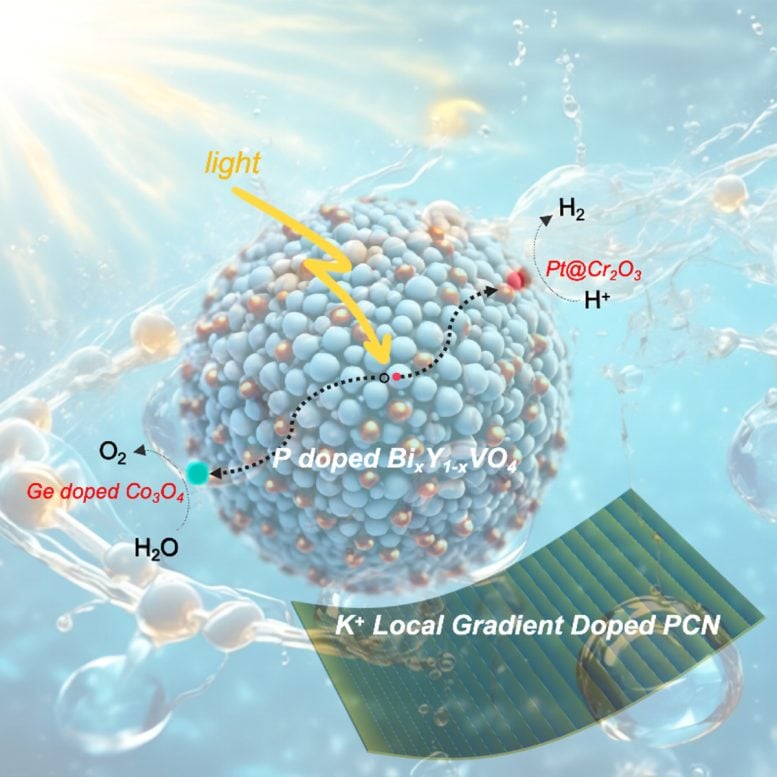
A comprehensive look at doping photocatalysts in water splitting under visible light is presented. Doping to regulate the band structure of Bi-based composite oxides achieves overall water splitting. Performance improvements in hydrogen production are made by doping to control the photocatalyst’s micro-morphology (selectively exposing high-activity crystal facets) and surface properties (surface phosphorization). A multi-local gradient doping technique constructs a three-dimensional potential well, efficiently driving charge separation, extending photogenerated carrier lifetimes, and enhancing photocatalytic water splitting performance. Credit: Chinese Journal of Catalysis
Photocatalytic water splitting, employing strategies like doping and defect control, has seen efficiency improvements, notably through recent advancements in doping methods that optimize energy conversion under visible light.
In photocatalytic water splitting, a photocatalyst, usually a semiconductor, absorbs light energy to drive the water splitting reaction. Upon absorbing light, the photocatalyst generates electron-hole pairs. The excited electrons reduce water, while the holes oxidize it.
However, there are several challenges associated with photocatalytic water splitting, mainly including low efficiency, limited visible light absorption, and photocorrosion of the photocatalyst. Thus, various strategies such as heterojunction formation, nanostructure design, cocatalysts utilization, dye sensitization, surface plasmonic enhancement, doping, and defect control are being explored to solve these problems and break the efficiency bottleneck.
Doping and Efficiency Improvements
Doping, in particular, has garnered significant attention. Various studies have demonstrated its efficacy. For instance, Kudo’s team achieved an apparent quantum yield (AQY) exceeding 50% through metal oxide modification. Nitrogen doping in TiO2, as reported by Asahi et al., proved crucial for band gap narrowing and enhanced photocatalytic activity. Domen et al. introduced a solid solution of gallium and zinc nitrogen oxide (Ga1–xZnx)(N1–xOx) for visible light water splitting. Chen et al. explored disorder introduction in TiO2 nanophase layers via hydrogenation to enhance solar absorption. Takata et al. achieved overall water splitting using a modified aluminum-doped strontium titanate (SrTiO3:Al) photocatalyst with an external quantum efficiency of up to 96%.
Recently, Prof. Wenfeng Shangguan’s team from Shanghai Jiao Tong University, China, integrated their research with other significant studies to provide a comprehensive review of energy band structure, microstructure, defect regulation, and doping strategies influencing photocatalytic activity. Their focus on doping rare earth elements into bismuth-based composite oxides aims to enhance the conduction band minimum and achieve overall water splitting under visible light. Their innovative asymmetric doping technique—Selected Local Gradient Doping—allows the controlled release of doped ions, promising significant contributions to novel materials exploration and energy conversion efficiency enhancement in photocatalytic water splitting under visible light.
Reference: “Account of doping photocatalyst for water splitting” by Wenjian Fang, Jiawei Yan, Zhidong Wei, Junying Liu, Weiqi Guo, Zhi Jiang and Wenfeng Shangguan, 22 May 2024, Chinese Journal of Catalysis.
DOI: 10.1016/S1872-2067(23)64637-6








Be the first to comment on "Breaking the Efficiency Bottleneck: The Power of Doping in Photocatalytic Water Splitting"