
Researchers have uncovered the molecular structure of Uncoupling protein 1 (UCP1), a protein instrumental in the burning of calories in brown fat tissue, often referred to as ‘good fat’. The essential molecular details discovered could aid in the development of therapeutics to activate UCP1 artificially, thus enabling the burning of excess calories and potentially combating obesity and diabetes.
Scientists have discovered the molecular structure of the protein UCP1, crucial to calorie burning in ‘good’ brown fat tissue. This breakthrough, providing detailed molecular insights, could enable the development of treatments that artificially activate UCP1, thus burning off excess calories to combat obesity and diabetes.
Researchers at the University of East Anglia and the University of Cambridge have made an important discovery in the race to find treatments for obesity and related diseases, such as diabetes.
A new study published today is the first to reveal the molecular structure of a protein called ‘Uncoupling protein 1’ (UCP1).
This protein allows brown fat tissue, or ‘good fat’, to burn off calories as heat — in contrast to conventional white fat that stores calories.
The breakthrough was made by an international collaboration between UEA, the University of Cambridge, the University of Pennsylvania, and the Free University of Brussels.
The team says that their findings provide crucial molecular details that will help develop therapeutics that activate UCP1 artificially to burn off excess calories from fat and sugar.
And that this could one-day combat obesity and related diseases, such as diabetes.
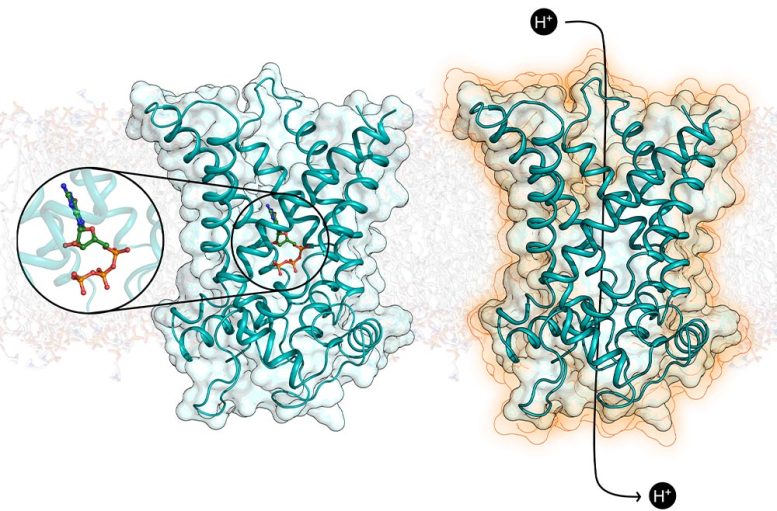
The human uncoupling protein in brown adipose tissue in its inactive form (left), inhibited by a nucleotide, and in its activated form (right), which short-circuits the mitochondrion to produce heat. Credit: Penn Medicine
Dr. Paul Crichton, from UEA’s Norwich Medical School, said: “As well as the conventional white fat that we are all familiar with, we can also develop brown fat.
“Brown fat is the good fat – it breaks down blood sugar and fat molecules to create heat and help maintain body temperature.
“Most of our fat, however, is white fat, which stores energy — and too much white fat leads to obesity.
“UCP1 is the key protein that allows the specialized brown fat to burn off calories as heat.
“We know that mammals switch on UCP1 activity in brown fat tissue to protect against the cold and to maintain body temperature — especially in new-borns, that cannot yet shiver to keep warm.
“Brown fat varies in humans, where it correlates with leanness in the population – and there has been a lot of interest in how to increase brown fat and activate UCP1 therapeutically, as a potential way to treat obesity.
“A lot of research has been focusing on finding ways to encourage brown fat and how to turn white fat into brown fat – in order to burn more calories and fight metabolic disease.
“But even with more brown fat – UCP1 must still be ‘switched on’ to gain full benefit. And research has been hampered by a lack of details on the molecular makeup of UCP1. Despite more than 40 years of research, we did not know what UCP1 looks like to understand how it works – until now.”
Lead researcher Prof Edmund Kunji, from the University of Cambridge, said: “Our paper reveals, for the first time, the structure of UCP1 in atomic detail, and how its activity in brown fat cells is inhibited by a key regulatory molecule.”
Using the Krios G3i, a cryogenic electron microscope at the Penn Singh Center for Nanotechnology, the team was able to view UCP1 in atomic detail.
“This is an exciting development that follows more than four decades of research into what UCP1 looks like and how it works,” said Vera Moiseenkova-Bell, an associate professor of Pharmacology and faculty director of the Beckman Center for Cryo-Electron Microscopy.
Prof Kunji said: “Our work shows how a regulator binds to prevent UCP1 activity, but more importantly the structure will allow scientists to rationalize how activating molecules bind to switch the protein on, leading to the burning of fat.
“The activated tissue can also remove glucose from the blood, which can help control diabetes.
“This is a significant breakthrough in this field,” he added.
“Structural basis of purine nucleotide inhibition of human uncoupling protein 1” is published in the journal Science Advances.
Reference: “Structural basis of purine nucleotide inhibition of human uncoupling protein 1” by Scott A. Jones, Prerana Gogoi, Jonathan J. Ruprecht, Martin S. King, Yang Lee, Thomas Zögg, Els Pardon, Deepak Chand, Stefan Steimle, Danielle M. Copeman, Camila A. Cotrim, Jan Steyaert, Paul G. Crichton, Vera Moiseenkova-Bell, Edmund R. S. Kunji, 31 May 2023, Science Advances.
DOI: 10.1126/sciadv.adh4251
This research was supported by the Medical Research Council, the Biological and Biotechnological Sciences Research Council, and by the National Institutes of Health/National Institute of General Medical Sciences. Nanobody discovery was funded by the Instruct-ERIC part of the European Strategy Forum on Research infrastructures, and the Research Foundation – Flanders, and the Strategic Research Program of the Vrije Universiteit Brussel.





Be the first to comment on "Flipping the Fat Burn Switch: The Breakthrough That Could Lead to New Obesity Treatments"