
Scientists have developed a novel photocatalyst system for efficient syngas production from methane steam reforming, using solar energy and operating under atmospheric pressure. This technology marks a significant step towards sustainable syngas production and a post-carbon energy future. Credit: SciTechDaily.com
A new solar-driven photocatalysis method for syngas production from methane steam reforming promises a more sustainable and efficient approach to syngas generation.
Recent research reveals a breakthrough in solar-driven syngas production, marking a potential transition to a post-carbon energy era. This innovative process involves the reforming of methane steam, a method that heats methane with steam in the presence of a catalyst to generate hydrogen and carbon monoxide, collectively known as syngas. Syngas is a valuable resource, serving as a versatile fuel.
Challenges in Methane Steam Reforming
Historically, achieving the necessary chemical reactions for methane steam reforming has been challenging. The process typically demands high temperatures between 700 and 1000 degrees Celsius and pressures exceeding 20 bar. These demanding conditions have limited its practicality and efficiency.
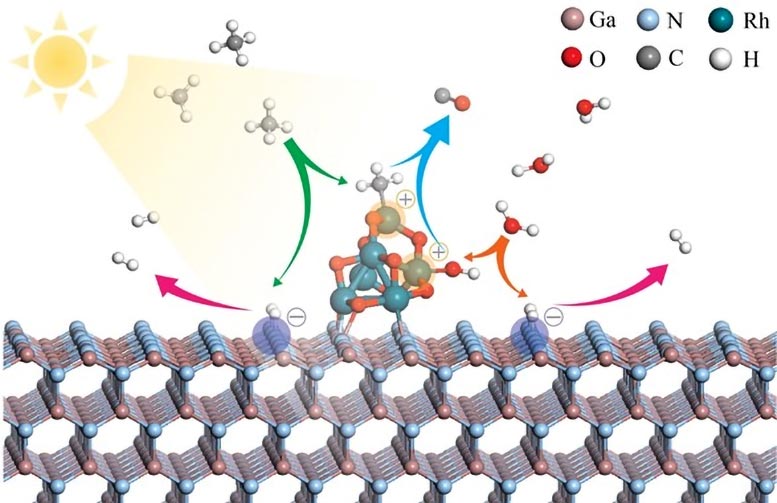
Schematic for simultaneous adsorption/activation of CH4 and H2O by RhOx/GaN system based on density functional theory calculations. Credit: Li et al.
Photocatalysis: A Novel Approach
Baowen Zhou and his team introduce a pioneering photocatalysis platform that enables syngas production in a quartz chamber under atmospheric pressure illuminated by a 300 W Xenon lamp without any other energy inputs. The core of this technology is based on group III nitride nanowires enhanced with rhodium nanoclusters.
Mechanism of the Photocatalytic Process
Detailed theoretical calculations, microscopic examinations, and in situ spectroscopic measurements have demonstrated that the RhOx/GaN@InGaN nanowires are capable of activating both methane and water molecules under light exposure. Just add light, and methane is split into methyl anions and hydrogen species, while water is split into hydrogen species and hydroxide. Subsequent reactions, facilitated by rhodium and gallium nitride, lead to the formation of syngas.
Efficiency and Stability of the New System
The effectiveness of this new method is evident, with a production rate of 8.1 mol syngas per gram of hydrogen and 10493 mol syngas per mol rhodium oxides observed over a 300-minute stability test. This represents a significant advancement in syngas production technology.
Reference: “A semiconducting hybrid of RhOx/GaN@InGaN for simultaneous activation of methane and water toward syngas by photocatalysis” by Dongke Li, Zewen Wu, Yixin Li, Xiaoxing Fan, S M Najib Hasan, Shamsul Arafin, Md Afjalur Rahman, Jinglin Li, Zhouzhou Wang, Tianqi Yu, Xianghua Kong, Lei Zhu, Sharif Md Sadaf and Baowen Zhou, 21 November 2023, PNAS Nexus.
DOI: 10.1093/pnasnexus/pgad347








Be the first to comment on "Sunlight to Syngas: Revolutionizing Methane Reforming"