
This is the Brazilain social wasp Polybia paulista. Credit: Prof. Mario Palma/Sao Paulo State University
A newly published study shows how Brazilian wasp venom selectively kills cancer cells without harming normal cells.
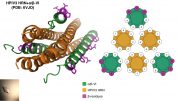
The social wasp Polybia paulista protects itself against predators by producing venom known to contain a powerful cancer-fighting ingredient. A Biophysical Journal study published September 1 reveals exactly how the venom’s toxin–called MP1 (Polybia-MP1)–selectively kills cancer cells without harming normal cells. MP1 interacts with lipids that are abnormally distributed on the surface of cancer cells, creating gaping holes that allow molecules crucial for cell function to leak out.
“Cancer therapies that attack the lipid composition of the cell membrane would be an entirely new class of anticancer drugs,” says co-senior study author Paul Beales, of the University of Leeds in the UK. “This could be useful in developing new combination therapies, where multiple drugs are used simultaneously to treat a cancer by attacking different parts of the cancer cells at the same time.”
MP1 acts against microbial pathogens by disrupting the bacterial cell membrane. Serendipitously, the antimicrobial peptide shows promise for protecting humans from cancer; it can inhibit the growth of prostate and bladder cancer cells, as well as multi-drug resistant leukemic cells. However, until now, it was not clear how MP1 selectively destroys cancer cells without harming normal cells.
Beales and co-senior study author João Ruggiero Neto of São Paulo State University in Brazil suspected that the reason might have something to do with the unique properties of cancer cell membranes. In healthy cell membranes, phospholipids called phosphatidylserine (PS) and phosphatidylethanolamine (PE) are located in the inner membrane leaflet facing the inside of the cell. But in cancer cells, PS and PE are embedded in the outer membrane leaflet facing the cell surroundings.
The researchers tested their theory by creating model membranes, some of which contained PE and/or PS, and exposing them to MP1. They used a wide range of imaging and biophysical techniques to characterize MP1’s destructive effects on the membranes. Strikingly, the presence of PS increased the binding of MP1 to the membrane by a factor of 7 to 8. On the other hand, the presence of PE enhanced MP1’s ability to quickly disrupt the membrane, increasing the size of holes by a factor of 20 to 30.
“Formed in only seconds, these large pores are big enough to allow critical molecules such as RNA and proteins to easily escape cells,” Neto says. “The dramatic enhancement of the permeabilization induced by the peptide in the presence of PE and the dimensions of the pores in these membranes was surprising.”
In future studies, the researchers plan to alter MP1’s amino acid sequence to examine how the peptide’s structure relates to its function and further improve the peptide’s selectivity and potency for clinical purposes. “Understanding the mechanism of action of this peptide will help in translational studies to further assess the potential for this peptide to be used in medicine,” Beales says. “As it has been shown to be selective to cancer cells and non-toxic to normal cells in the lab, this peptide has the potential to be safe, but further work would be required to prove that.”
Reference: “PE and PS Lipids Synergistically Enhance Membrane Poration by a Peptide with Anticancer Properties” by Natália Bueno Leite, Anders Aufderhorst-Roberts, Mario Sergio Palma, Simon D. Connell, João Ruggiero Neto and Paul A. Beales, 1 September 2015, Biophysical Journal.
DOI:10.1016/j.bpj.2015.07.033






Be the first to comment on "Brazilian Wasp Venom Kills Cancer Cells Without Harming Normal Cells"