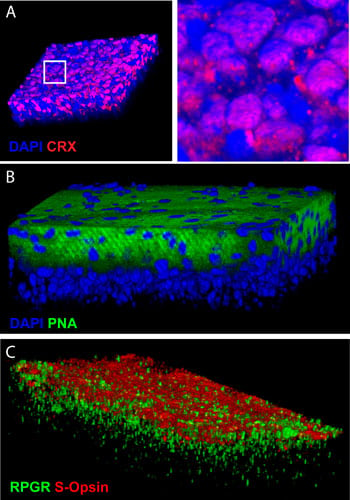
Researchers have succeed in producing photoreceptors from human embryonic stem cells. This image illustrates the 3D reconstruction of the tissue that was produced in vitro and labeled with antibodies against photoreceptor-specific proteins. Credit: G. Bernier, Université de Montréal
By producing photoreceptors from human embryonic stem cells, researchers from the University of Montreal have taken a major step forward in the fight against age-related macular degeneration.
Age-related macular degeneration (AMRD) could be treated by transplanting photoreceptors produced by the directed differentiation of stem cells, thanks to findings published today by Professor Gilbert Bernier of the University of Montreal and its affiliated Maisonneuve-Rosemont Hospital. ARMD is a common eye problem caused by the loss of cones. Bernier’s team has developed a highly effective in vitro technique for producing light-sensitive retina cells from human embryonic stem cells. “Our method has the capacity to differentiate 80% of the stem cells into pure cones,” Professor Gilbert explained. “Within 45 days, the cones that we allowed to grow towards confluence spontaneously formed organized retinal tissue that was 150 microns thick. This has never been achieved before.”
In order to verify the technique, Bernier injected clusters of retinal cells into the eyes of healthy mice. The transplanted photoreceptors migrated naturally within the retina of their host. “Cone transplant represents a therapeutic solution for retinal pathologies caused by the degeneration of photoreceptor cells,” Bernier explained. “To date, it has been difficult to obtain great quantities of human cones.” His discovery offers a way to overcome this problem, offering hope that treatments may be developed for currently non-curable degenerative diseases, like Stargardt disease and ARMD. “Researchers have been trying to achieve this kind of trial for years,” he said. “Thanks to our simple and effective approach, any laboratory in the world will now be able to create masses of photoreceptors. Even if there’s a long way to go before launching clinical trials, this means, in theory, that will eventually be able to treat countless patients.”
The findings are particularly significant in light of improving life expectancies and the associated increase in cases of ARMD. ARMD is in fact the greatest cause of blindness in people over the age of 50 and affects millions of people worldwide. And as we age, it is more and more difficult to avoid – in people over 80, this accelerated aging of the retina affects nearly one in four. People with ARMD gradually lose their perception of colors and details to the point that they can no longer read, write, watch television, or even recognize a face.
ARMD is due to the degeneration of the macula, which is the central part of the retina that enables the majority of eyesight. This degeneration is caused by the destruction of the cones and cells in the retinal pigment epithelium (RPE), a tissue that is responsible for the reparation of the visual cells in the retina and for the elimination of cells that are too worn out. However, there is only so much reparation that can be done as we are born with a fixed number of cones. They therefore cannot naturally be replaced. Moreover, as we age, the RPE’s maintenance is less and less effective – waste accumulates, forming deposits. “Differentiating RPE cells is quite easy. But in order to undertake a complete therapy, we need neuronal tissue that links all RPE cells to the cones. That is much more complex to develop,” Bernier explains, noting nonetheless that he believes his research team is up to the challenge.
Bernier has been interested in the genes that code and enable the induction of the retina during embryonic development since completing his Ph.D. in Molecular Biology in 1997. “During my post-doc at the Max-Planck Institute in Germany, I developed the idea that there was a natural molecule that must exist and be capable of forcing embryonic stem cells into becoming cones,” he said. Indeed, bioinformatic analysis led him to predict the existence of a mysterious protein: COCO, a “recombinational” human molecule that is normally expressed within photoreceptors during their development.
In 2001, he launched his laboratory at Maisonneuve-Rosemont Hospital and immediately isolated the molecule. But it took several years of research to demystify the molecular pathways involved in the photoreceptors’ development mechanism. His latest research shows that in order to create cones, COCO can systematically block all the signaling pathways leading to the differentiation of the other retinal cells in the eye. It’s by uncovering this molecular process that Bernier was able to produce photoreceptors. More specifically, he has produced S-cones, which are photoreceptor prototypes that are found in the most primitive organisms.
Beyond the clinical applications, Professor Bernier’s findings could enable the modeling of human retinal degenerative diseases through the use of induced pluripotent stem cells, offering the possibility of directly testing potential avenues for therapy on the patient’s own tissues.
Reference: “Differentiation of human embryonic stem cells into cone photoreceptors through simultaneous inhibition of BMP, TGFβ and Wnt signaling” by Shufeng Zhou, Anthony Flamier, Mohamed Abdouh, Nicolas Tétreault, Andrea Barabino, Shashi Wadhwa and Gilbert Bernier, 6 August 2015, Development.
DOI: 10.1242/dev.125385





Be the first to comment on "Scientists Produce Photoreceptors from Embryonic Stem Cells"