Scientists have developed a new technique called phosphorus fluoride exchange (PFEx), expanding the realm of click chemistry by using phosphorous as a chemical connector to construct complex molecules. This advancement could potentially contribute to the discovery of effective cancer therapeutics and the creation of new materials with beneficial properties like flame retardance or antimicrobial effects, while maintaining environmental sustainability as phosphorous bonds can be broken down easily during recycling.
Diversity is an essential path to discovery for chemists like Professor John Moses of Cold Spring Harbor Laboratory (CSHL). The broader the spectrum of molecules they investigate, the higher the chances of identifying something of value. Moses’ lab’s recent progress allows them to rapidly construct an expansive variety of complex molecules. Moses is hopeful that among these molecules, there will be new and effective cancer treatments.
Together with K. Barry Sharpless, a two-time Nobel laureate, Moses’ lab has developed a chemical transformation called phosphorus fluoride exchange, or PFEx. PFEx efficiently snaps together chemical building blocks to form new molecules, in a reliable process known as click chemistry. Click chemistry already offers chemists a powerful set of tools. As the newest addition to that tool kit, PFEx takes a cue from biology and uses phosphorous as a chemical connector.

Shoujun Sun, seen here, is a postdoctoral fellow in Cold Spring Harbor Laboratory Professor John Moses’ lab. Sun led a new Moses lab study that marks a significant breakthrough for the field of click chemistry. Credit: Moses lab/Cold Spring Harbor Laboratory
Inside cells, phosphorous gives structure to DNA and holds together essential energy-storing molecules. It’s a versatile connector. It can readily connect multiple chemical groups. These groups can be arranged around the phosphorous hub to create three-dimensional shapes.
Moses says: “Nature has recognized its importance—it’s a privileged group. If we’re trying to make drugs that interact with biology, we should not ignore that fact.”
Chemists can now use PFEx to click together multiple different chemical components around a single phosphorous hub. By incorporating more phosphorous connectors, they can build even more complex molecules. “We’re now decorating this three-dimensional linkage. And that’s going to allow us to access some new chemical space,” says CSHL Research Investigator Joshua Homer. “When you access new space, you’re accessing new function.”
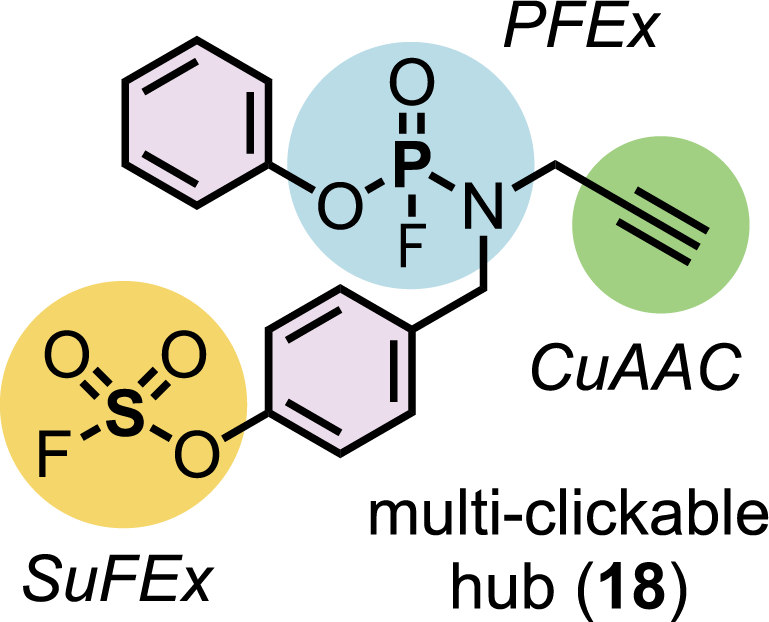
The power and potential of PFEx-based chemistry lie in its ability to rapidly and reliably click together complex molecules using sustainable lab science. The illustration above shows how PFEx is compatible with other click chemistry bonds, including the 2022 Nobel prize-winning CuAAC reactions. Credit: Moses lab/Cold Spring Harbor Laboratory
PFEx reactions might even enable drugs to latch onto their targets inside the body. Moses’ team has already begun exploring PFEx as a source of cancer therapeutics. One benefit to this approach is that researchers can optimize the reactivity of the molecules involved in PFEx reactions. This could ensure potential drugs interact only with their desired targets, reducing the risk of side effects.
The researchers expect their new kind of click chemistry will help create materials with useful properties. For example, PFEx might be used to incorporate flame retardants or antimicrobials into new surfaces. Moses says PFEx materials will have an important advantage over the “forever chemicals” found in many of today’s products. Phosphorous bonds are not excessively stable. This means they can be easily broken down when a product is ready for recycling.
Reference: “Phosphorus fluoride exchange: Multidimensional catalytic click chemistry from phosphorus connective hubs” by Shoujun Sun, Joshua A. Homer, Christopher J. Smedley, Qing-Qing Cheng, K. Barry Sharpless and John E. Moses, 7 June 2023, Chem.
DOI: 10.1016/j.chempr.2023.05.013
The study was funded by the National Cancer Institute, Cold Spring Harbor Laboratory, Northwell Health, the F. M. Kirby Foundation, the Sunshine Foundation, S. J. Edwards, the Starr Foundation, The Wasily Family Foundation, La Trobe University, and the National Institutes of Health.







Be the first to comment on "Scientists Harness Biology’s Favorite Chemical"