
Structural biologists have captured images of the CALHM1 channel in human neurons, gaining insights into its function and structure. This research could help understand CALHM1’s role in Alzheimer’s disease and taste perception, providing a basis for potential drug development.
Researchers at Cold Spring Harbor Laboratory have generated detailed images of a neuronal channel known as Calcium Homeostasis Modulator Protein 1 (CALHM1), which plays significant roles in various physiological processes, including taste perception and possibly Alzheimer’s disease regulation. The research highlights the structure and functionality of the CALHM1 channel and emphasizes the role of phospholipids in stabilizing the channel. The findings offer a foundation for further exploration into CALHM1’s role in human health and its potential as a drug target.
The Role of CALHM1 Protein in Neurons
The human body’s neurons are speckled with tiny pores that enable the passage of essential molecules into and out of our cells. These channels are vital for neurons to transmit signals that facilitate our movement, cognition, and perception of the world. Recently, structural biologists at Cold Spring Harbor Laboratory (CSHL) have acquired unprecedented images of one of the most sizable pores present in human neurons, known as the Calcium Homeostasis Modulator Protein 1, or CALHM1.
CALHM1 and Alzheimer’s Disease
Past studies suggest that mutations in the Cahlm1 gene might elevate the risk for Alzheimer’s disease. The latest research from CSHL offers new insights into how the CALHM1 channel operates in humans and the ways in which it can become obstructed.
Functions of CALHM1
CSHL Professor Hiro Furukawa and postdoctoral researcher Johanna Syrjänen have devoted several years to the study of CALHM1, which appears to be implicated in a broad array of physiological processes.
In our tongues, CALHM1 contributes to our perception of tastes like sweet, sour, or umami. In our brains, it may play a part in regulating the accumulation of a plaque-forming protein linked to Alzheimer’s.
Structure and Regulation of CALHM1
Furukawa, Syrjänen, and their team employed a technique known as cryo-electron microscopy to produce detailed three-dimensional images of the human CALHM1 channel.
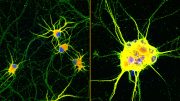
These images illustrate how eight copies of the CALHM1 protein come together to form the circular channel. Each protein features a flexible appendage that extends into the pore, potentially managing its opening and closing, a characteristic that Syrjänen equates to “octopus tentacles.”
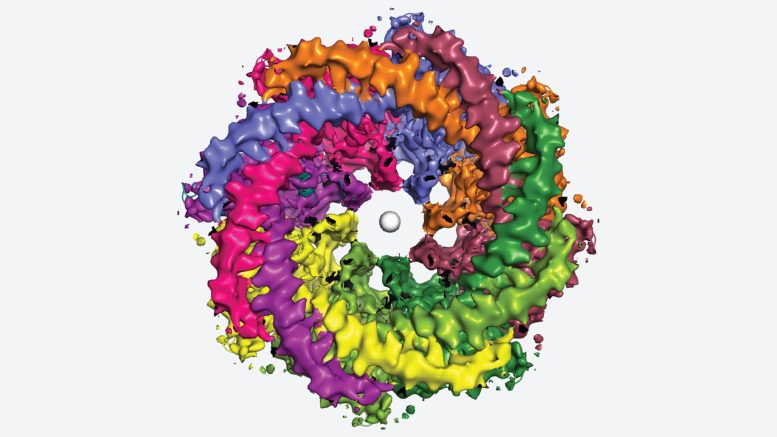
Cryo-electron microscopy reveals that the human CALHM1 channel has an eight-protein assembly pattern, similar to that found in chickens. Note the number of colored arm-like structures above. The dot at the center of the image is ruthenium red, a chemical researchers use to block off the channel. Credit: Furkawa lab/Cryo-EM Facility/Cold Spring Harbor Laboratory
The researchers also discovered that fatty molecules, phospholipids, are crucial for stabilizing and controlling this eight-part channel. These important fats are abundant in eggs, cereal, lean meats, and seafood.
Additionally, the team demonstrated how a chemical commonly used by researchers to block CALHM1 can become lodged in the channel, a finding that could prove beneficial for potential drug development targeting CALHM1.
Future Implications of CALHM1 Research
Syrjänen says: “If you are thinking way down the line, ‘Can we control taste perception or influence this protein?’ we now know one of the places where you could block the protein activity.”
Syrjänen notes that the human CALHM1 channel looks a lot like the version she and Furukawa studied in chickens in 2020. However, determining the structure of the human protein proved more technically challenging. But, researchers agree, it’s crucial to gain a deeper understanding of the channel’s impact on human health.
“There are numerous unanswered questions surrounding CALHM1,” Furukawa says. For example, how does the energy-carrying molecule, ATP, escape from cells via this channel? And could this trigger the body’s inflammatory response? “Our research group will continue unraveling this vital molecular machine to better understand the CALHM1 channel’s functionality.”
Reference: “Structure of human CALHM1 reveals key locations for channel regulation and blockade by ruthenium red” by Johanna L. Syrjänen, Max Epstein, Ricardo Gómez and Hiro Furukawa, 28 June 2023, Nature Communications.
DOI: 10.1038/s41467-023-39388-3
Funding: NIH/National Institutes of Health, Austin’s Purpose, Robertson Research Fund, Doug Fox Alzheimer’s Fund, Heartfelt Wings Foundation, Gertrude and Louis Feil Family Trust, Charles H. Revson Senior Fellowship in Biomedical Science.





Be the first to comment on "Taste Detection Unmasked: The Eight-Armed Octopus Pore in Our Neurons"