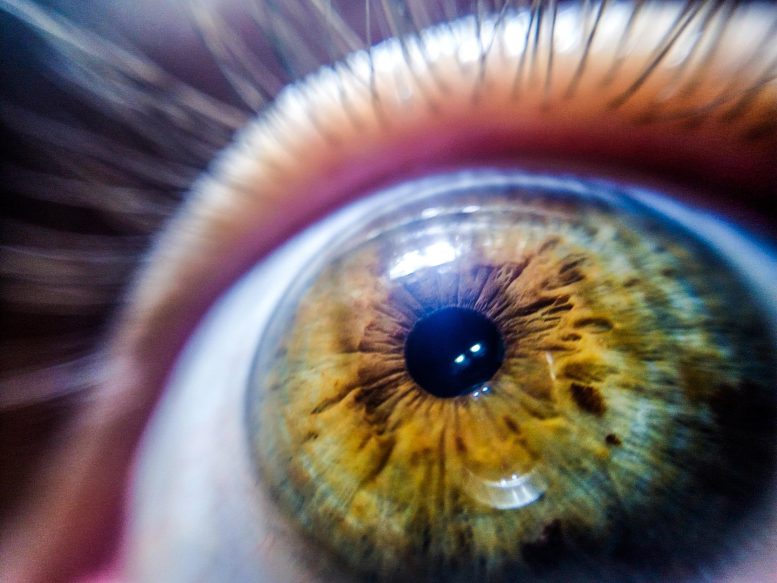
After episodes of sleep deprivation, eye problems such as dryness and itching are common. Long-term sleep deprivation can increased the risk for eye disease.
Sleep deprivation, which means getting too little high-quality sleep, is a serious health problem. More than one-third of Americans report sleeping less than the suggested minimum of seven hours every night. Sleep deprivation has negative impacts on mental and physical health. Eye problems such as dryness and itching are commonly experienced after episodes of sleep deprivation, while long-term sleep deprivation comes with an increased risk for eye disease.
The cornea is the transparent front part of the eye that covers the iris, pupil, and anterior chamber. Along with the anterior chamber and lens, the cornea refracts light, accounting for approximately two-thirds of the eye’s total optical power. LASIK and other surgical techniques can reshape the cornea.
The cornea, which is the transparent tissue layer covering the eye, is essential for assuring health and function of the eye. The cornea is maintained by stem cells, which divide to replace dying cells and to repair small injuries. Corneal stem cell activity needs to be precisely tuned to assure an adequate output of new corneal cells, and dysregulation of corneal stem cells can lead to eye disease and impaired vision.
In a research study published on April 28, 2022, in Stem Cell Reports, researchers Wei Li, Zugou Liu, and colleagues from Xiamen University, China, and Harvard Medical School, USA, evaluated how sleep deprivation impacts corneal stem cells. Their experiments in mice showed that short-term sleep deprivation increased the rate at which stem cells in the cornea multiplied. At the same time, sleep deprivation altered the composition of the protective tear film, reducing the tear film antioxidants in sleep-deprived mice. The researchers found that the tear film composition had a direct impact on corneal stem cell activity and, encouragingly, application of eye drops containing antioxidants reversed the excessive stem cell activity.

The microenvironment of the tear film is just like the macroenvironment of the sea. It protects the corneal basal epithelial stem cells from overexposing to environmental Reactive Oxygen Species (ROS). There are many antioxidants present in the tear film, like the diversity of marine creatures. Even a short period of sleep deprivation will disrupt the balance of the redox homeostasis in the tear film, thus affecting the behavior of underneath corneal epithelial stem cells. Credit: Illustration by Yuehang Li
The study revealed that serious effects on corneal health, such as thinning and ruffling of the cornea and loss of transparency, were seen after long-term sleep deprivation. Further, corneas of long-term sleep-deprived mice contained fewer stem cells, suggesting that persistent stimulation of stem cell activity over longer periods led to exhaustion and loss of corneal stem cells.
These data suggest that sleep deprivation negatively affects the stem cells in the cornea, possibly leading to vision impairment in the long run. Further studies are required to confirm that similar processes are happening in human corneal stem cells and in patients, and to test if local antioxidant therapy may overcome some of the negative effects of sleep deprivation on corneal health.
Reference: “Sleep deprivation induces corneal epithelial progenitor cell over-expansion through disruption of redox homeostasis in the tear film” by Sanming Li, Liying Tang, Jing Zhou, Sonia Anchouche, Dian Li, Yiran Yang, Zhaolin Liu, Jieli Wu, Jiaoyue Hu, Yueping Zhou, Jia Yin, Zuguo Liu and Wei Li, 28 April 2022, Stem Cell Reports.
DOI: 10.1016/j.stemcr.2022.03.017






Be the first to comment on "Sleep Deprivation Impairs Stem Cells in the Cornea – Possibly Leading to Vision Impairment"