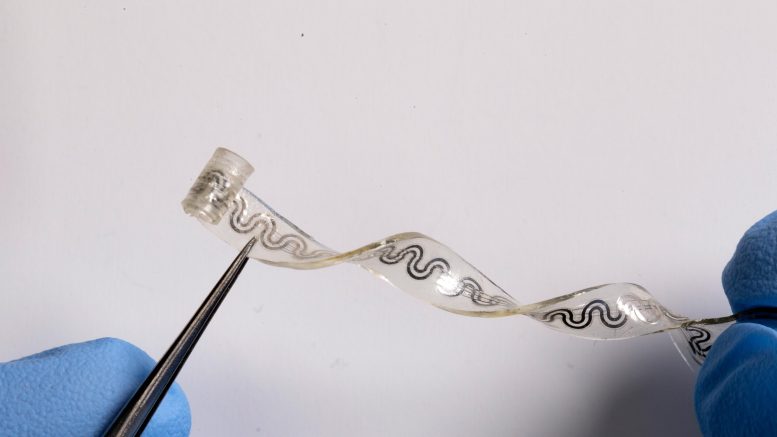
The soft flexible device bends and stretches with the body, without the need for bulky, rigid hardware. Credit: Northwestern University
New device has the potential to provide an alternative to opioids and other highly addictive drugs.
A small, soft, flexible implant that relieves pain on demand and without the use of drugs has been developed by a team of researchers led by Northwestern University. The first-of-its-kind device could provide a much-needed alternative to opioids and other highly addictive medications.
By softly wrapping around nerves, the biocompatible, water-soluble device is able to deliver precise, targeted cooling, which numbs nerves and blocks pain signals to the brain. With an external pump, the user can remotely activate the device and then increase or decrease its intensity. After the device is no longer needed, it naturally absorbs into the body. This bypasses the need for surgical extraction.
According to the researchers, the device has the potential to be most valuable for patients who undergo routine surgeries or even amputations that commonly require post-operative medications. Surgeons could implant the device during the procedure to help manage the patient’s post-operative pain.
The paper describes the device’s design and demonstrates its effectiveness in an animal model. It was published in the July 1 issue of the journal Science.
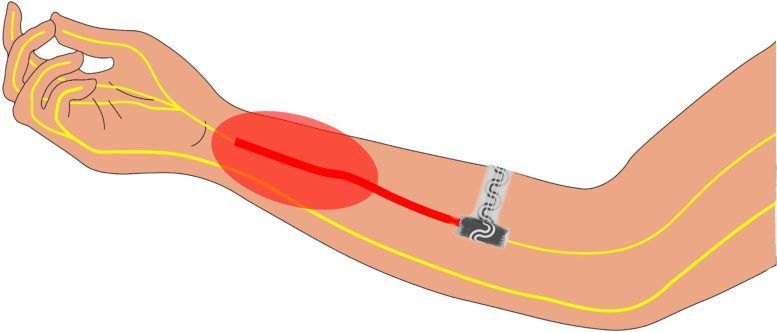
Illustration of the implantable device inside an arm. The red oval indicates pain. The device softly wraps around the peripheral nerve to silence signals to the brain. Credit: Northwestern University
“Although opioids are extremely effective, they also are extremely addictive,” said Northwestern’s John A. Rogers, who led the development of the device. “As engineers, we are motivated by the idea of treating pain without drugs — in ways that can be turned on and off instantly, with user control over the intensity of relief. The technology reported here exploits mechanisms that have some similarities to those that cause your fingers to feel numb when cold. Our implant allows that effect to be produced in a programmable way, directly and locally to targeted nerves, even those deep within surrounding soft tissues.”
A bioelectronics pioneer, John A. Rogers is the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery in the McCormick School of Engineering and Northwestern University Feinberg School of Medicine. He is also the founding director of the Querrey Simpson Institute for Bioelectronics. Jonathan Reeder, the paper’s first author, is a former Ph.D. candidate in Rogers’ laboratory.
How it works
Although the new device might sound like science fiction, it actually leverages a simple, common concept that everyone knows: evaporation. Similar to how evaporating sweat cools the body, the device contains a liquid coolant that is induced to evaporate at the specific location of a sensory nerve.
“As you cool down a nerve, the signals that travel through the nerve become slower and slower — eventually stopping completely,” said study co-author Dr. Matthew MacEwan of Washington University School of Medicine in St. Louis. “We are specifically targeting peripheral nerves, which connect your brain and your spinal cord to the rest of your body. These are the nerves that communicate sensory stimuli, including pain. By delivering a cooling effect to just one or two targeted nerves, we can effectively modulate pain signals in one specific region of the body.”
Watch the device slowly dissolve over the course of 50 days. The device breaks down into biocompatible components, which are then naturally absorbed into the body. Credit: Northwestern University
To induce the cooling effect, the device contains tiny microfluidic channels. One channel contains the liquid coolant (perfluoropentane), which is already clinically approved as an ultrasound contrast agent and for pressurized inhalers. A second channel contains dry nitrogen, an inert gas. When the liquid and gas flow into a shared chamber, a reaction occurs that causes the liquid to promptly evaporate. Simultaneously, a tiny integrated sensor monitors the temperature of the nerve to ensure that it’s not getting too cold, which could cause tissue damage.
“Excessive cooling can damage the nerve and the fragile tissues around it,” Rogers said. “The duration and temperature of the cooling must therefore be controlled precisely. By monitoring the temperature at the nerve, the flow rates can be adjusted automatically to set a point that blocks pain in a reversible, safe manner. On-going work seeks to define the full set of time and temperature thresholds below which the process remains fully reversible.”
Precision power
While other cooling therapies and nerve blockers have been tested experimentally, all have significant limitations that the new device overcomes. Scientists have previously explored cryotherapies, for example, which are injected with a needle. Instead of targeting specific nerves, these imprecise approaches cool large areas of tissue, potentially leading to unwanted effects such as inflammation and tissue damage.
At its widest point, Northwestern’s tiny device is just 5 millimeters (0.2 inches) wide. One end is curled into a cuff that softly wraps around a single nerve, bypassing the need for sutures. By precisely targeting only the affected nerve, the device spares surrounding regions from unnecessary cooling, which could lead to side effects.
“You don’t want to inadvertently cool other nerves or the tissues that are unrelated to the nerve transmitting the painful stimuli,” MacEwan said. “We want to block the pain signals, not the nerves that control motor function and enable you to use your hand, for example.”
Previous researchers also have explored nerve blockers that use electrical stimulation to silence painful stimuli. These, too, have limitations.
“You can’t shut down a nerve with electrical stimulation without activating it first,” MacEwan said. “That can cause additional pain or muscle contractions and is not ideal, from a patient’s perspective.”
Disappearing act
This new technology is the third example of bioresorbable electronic devices from the Rogers lab, which introduced the concept of transient electronics in 2012, published in Science. In 2018, Rogers, MacEwan, and colleagues demonstrated the world’s first bioresorbable electronic device — a biodegradable implant that speeds nerve regeneration, published in Nature Medicine. Then, in 2021, Rogers and colleagues introduced a transient pacemaker, published in Nature Biotechnology.
All components of the devices are biocompatible and naturally absorb into the body’s biofluids over the course of days or weeks, without needing surgical extraction. The bioresorbable devices are completely harmless — similar to absorbable stitches.
At the thickness of a sheet of paper, the soft, elastic nerve-cooling device is ideal for treating highly sensitive nerves.
“If you think about soft tissues, fragile nerves, and a body that’s in constant motion, any interfacing device must have the ability to flex, bend, twist, and stretch easily and naturally,” Rogers said. “Furthermore, you would like the device to simply disappear after it is no longer needed, to avoid delicate and risky procedures for surgical removal.”
Reference: “Soft, bioresorbable coolers for reversible conduction block of peripheral nerves” by Jonathan T. Reeder, Zhaoqian Xie, Quansan Yang, Min-Ho Seo, Ying Yan, Yujun Deng, Katherine R. Jinkins, Siddharth R. Krishnan, Claire Liu, Shannon McKay, Emily Patnaude, Alexandra Johnson, Zichen Zhao, Moon Joo Kim, Yameng Xu, Ivy Huang, Raudel Avila, Christopher Felicelli, Emily Ray, Xu Guo, Wilson Z. Ray, Yonggang Huang, Matthew R. MacEwan and John A. Rogers, 30 June 2022, Science.
DOI: 10.1126/science.abl8532
The study was supported by the Phil and Penny Knight Campus for Accelerating Scientific Impact, the Querrey Simpson Institute for Bioelectronics, and the National Science Foundation (award number CMM1635443).







What a nice tool to make combat personnel injury tolerant.
Great for elderly. Arthritis etc.
clever. The wires and temp sensor dissolve also? More likely they get pulled out thru the entrance after the channels break down. Temp control at the nerve – well done.
Will this be effect for a three time fusion and l4 root nerve decompression. My hip is in pain unless setting. I have tried to walk and or run but with little success
When and where will it be available?
Migraine treatment. This would be so great, except we wouldn’t want it to dissolve!
Will this work on Severe Chronic Pelvic pain? Who would we see to get this. The Gynecologist or the pain clinic that is untrustworthy? I would only see my Gynecologist or a specialist with this device. Thanks
Does this work for sciatica and sacroiliac dysfunction
Yes. Will Medicare pay for it? Or is it an elective solution available only to people who can pay cash for it?
What happens after it dissolves? Would you then have to get a new device implanted?
Sounds like a solution for surgical pain, not for continues pain. I had back pain for over 20 years. I had 5 dics removed and now have arthritis in my back. Have been on Ultraman since 2006. Need something that is more permanent. After all isn’t that (chronic pain) what gets people addicted.
Sign me up. Chronic pain everywhere for years and getting worse all the time. I’m only 64. It’s from OA, fibro, heart disease and Crohn’s ..
Could this work for chronic headache sufferers like myself? Has there been any thought into making these so that they stay inside us forever or over a longer period or does it have to dissolve?
I agree with Ronaldo, this sounds like it would be wonderful for post-surgical pain. However, how would you apply it to chronic pain? There are millions of people living in severe, chronic pain. Yhis is designed to dissolve, not continue working long-term. Would someone with long-term pain need to have another one implanted? Or would they just not be a candidate for this product? I also like to know more about the materials it is made of, and what the side effects are of them solving in our bodies. Also, how are the wires removed? Thank you.
I have a son that was shot when he was 17,he is 30 now. And has nerve pains every 15 min. This would be good for him. Can I get some info on who to contact? Please.
Unfortunate for chronic pain sufferers like myself. Would be great if it could replace Spinal Chord Stimulators which are so dangerous & have so many problems for the patient. What a novel idea this is though & dissolves in 7 weeks,pretty amazing technology, I give them an A+
Maybe within 50 years they will release it. Medicine is freaking slow.
I would like to know how to get on a list for a trial. I would be willing to be a test subject as to the effectiveness of the procedure. It sure is worth a try to avoid carterizing and or lower lumbar fusion
I am desperate to stop the spine pain, from the surgery that didn’t help! I have RA, Fibromyalgia DDD, 24-7 with muscle spasms-list of others! I am in desperate need of pain relief 🥲!