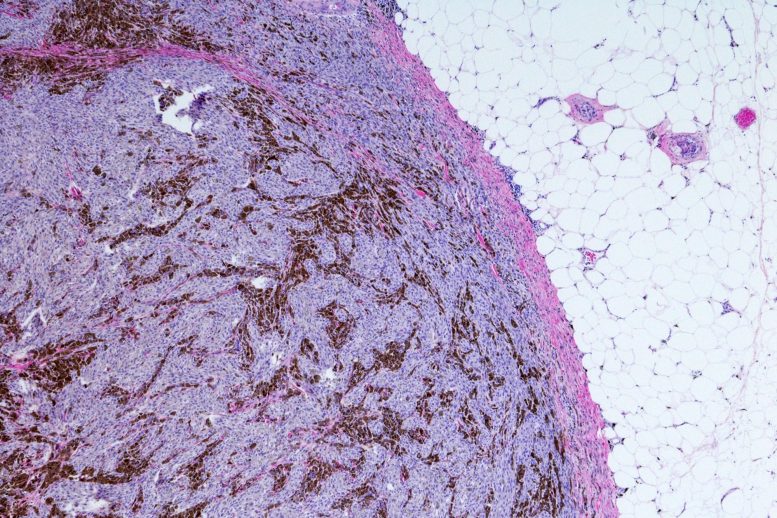
Metastatic Malignant Melanoma (Skin Cancer) Micrograph
New research shows that the approved drug Zelboraf nearly doubles the median survival time for patients with metastatic melanoma. By following 132 patients, researchers found that those taking Zelboraf, which blocks a mutated BRAF protein, survived an average of 15.9 months versus the typical survival of about nine months without Zelboraf.
Researchers from the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles (UCLA), together with scientists from 12 other sites in the U.S. and Australia, report for the first time that a newly approved drug for metastatic melanoma nearly doubles the median survival time for patients with a common genetic mutation — a finding that will change the way this deadly form of skin cancer is treated.
The data comes from an international Phase 2 study of the drug Zelboraf that included 132 patients who were followed for at least one year.

Dr. Antoni Ribas. Credit: UCLA
Patients with this advanced form of melanoma, in which the cancer has spread to other organs, typically survive about nine months. Those taking Zelboraf, which blocks a mutated BRAF protein, survived an average of 15.9 months, according to the study’s senior author, Dr. Antoni Ribas, a professor of hematology–oncology and a researcher at the Jonsson Cancer Center.
“This study shows that Zelboraf changes the natural history of this disease,” Ribas said. “This data is beyond what I would have expected. We’re seeing a significant number of patients with durable responses to the drug, and that the whole group of treated patients is living longer. These results tell us that this drug is having a very big impact, and this changes the way we treat metastatic melanoma.”
The study appears on February 23 in the peer-reviewed New England Journal of Medicine.
About 50 percent of patients with metastatic melanoma — or some 4,000 people a year — have the BRAF mutation and can be treated with Zelboraf, two pills taken twice daily, Ribas said. Of those, 53 percent have an objective response to the drug, meaning their tumors shrink by more than 30 percent. An additional 30 percent of patients have tumor responses of lesser magnitude. Only 14 percent of patients with the BRAF mutation failed to respond to Zelboraf.
The drug represents a breakthrough in treating metastatic melanoma. Prior to this, 10 percent or less of patients with this advanced form of the disease responded to any of the available conventional treatments, Ribas said.
“We knew this drug would make the melanomas shrink in a large proportion of patients and that it worked better than chemotherapy,” Ribas said. “This study confirmed that patients taking Zelboraf are living longer.”
The main limitation of Zelboraf is that tumors eventually become resistant to the drug. But Jonsson Cancer Center researchers are studying this resistance and have uncovered several mechanisms by which the cancer gets around Zelboraf. They currently are seeking agents to target those mechanisms, Ribas said.
For Louise Belley, 60, of Santa Ana, California, Zelboraf has allowed her to live much longer than her doctors initially predicted, and last June, she was able to see her daughter graduate from college. Belley was diagnosed with metastatic melanoma in November 2007. In June 2009, she was among the first people to get the drug when it was in Phase 1 studies at the Jonsson Cancer Center.
Her first CT scan, in September 2009 (after joining the trial nearly two and a half years ago), was clear of any cancer, she said. Her scans remain clean today.
“At first, I was devastated by my diagnosis, because I realized I was in a really bad place,” Belley said. “But now I feel strongly that the chances are good that I will continue to be cancer-free, and I plan to live every day to the fullest.”
Zelboraf was approved by the U.S. Food and Drug Administration for use in metastatic melanoma in August 2011. About 70,000 new cases of melanoma are diagnosed each year in the U.S. Of those individuals, 8,000 will die of the disease.
“This trial shows a high rate of response to [Zelboraf] in patients with metastatic melanoma and activating BRAF mutations,” the study states. “These results independently confirm the high response rate and response duration shown in a Phase I trial.”
Reference: “Survival in BRAF V600–Mutant Advanced Melanoma Treated with Vemurafenib” by Jeffrey A. Sosman, M.D.; Kevin B. Kim, M.D.; Lynn Schuchter, M.D.; Rene Gonzalez, M.D.; Anna C. Pavlick, D.O.; Jeffrey S. Weber, M.D., Ph.D.; Grant A. McArthur, M.B., B.S., Ph.D.; Thomas E. Hutson, D.O.; Stergios J. Moschos, M.D.; Keith T. Flaherty, M.D.; Peter Hersey, F.R.A.C.P., D.Phil.; Richard Kefford, M.B., B.S., Ph.D.; Donald Lawrence, M.D.; Igor Puzanov, M.D.; Karl D. Lewis, M.D.; Ravi K. Amaravadi, M.D.; Bartosz Chmielowski, M.D., Ph.D.; H. Jeffrey Lawrence, M.D.; Yu Shyr, Ph.D.; Fei Ye, Ph.D.; Jiang Li, Ph.D.; Keith B. Nolop, M.D.; Richard J. Lee, M.D.; Andrew K. Joe, M.D. and Antoni Ribas, M.D., Ph.D., 23 February 2012, New England Journal of Medicine.
DOI: 10.1056/NEJMoa1112302








Be the first to comment on "Zelboraf Nearly Doubles Median Survival Time for Patients with Metastatic Melanoma"