Understanding the connections between different brain regions could lead to better treatment options for conditions like Alzheimer’s, schizophrenia, and depression. In 2019, a technique known as BARseq was developed to map these connections by identifying brain cells through the genes they express and tracing their neural circuitry. Initially capable of mapping thousands of pathways using RNA “barcodes,” this technique has now been enhanced to map millions of neurons. The research has expanded into the visual cortex, investigating how brain function changes when neural pathways are disrupted, providing deeper insights into brain development and functioning.
Researchers developed and enhanced BARseq, a technique to map brain cell connections by gene expression, aiming to improve treatments for neurological conditions. They discovered that blindness alters visual cortex gene expression, and ongoing work seeks to expand BARseq’s capabilities to understand brain connectivity and development.
Exploring how different areas of the brain interact could lead to improved treatments for conditions such as Alzheimer’s, schizophrenia, and depression.
In 2019, as a postdoc in Cold Spring Harbor Laboratory’s (CSHL’s) Zador lab, Xiaoyin Chen helped develop a technique to map these connections. BARseq identifies cells in the brain by the genes they use and traces the connecting neural circuitry. Early versions of BARseq mapped gene expression across thousands of neural pathways, using “barcodes” or short snippets of RNA.
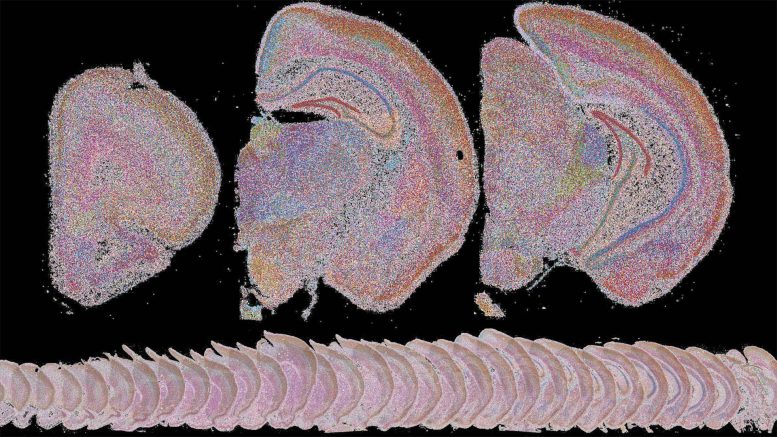
“I think of BARseq’s maps as sort of like a painting aid,” says former CSHL postdoc Xiaoyin Chen. “All these little dots make up shapes. And you can actually zoom in and look at different parts and distinguish different cell types.” Credit: Chen lab/Allen Institute for Brain Science
Chen is now an assistant investigator at the Allen Brain Institute. He recently reunited with CSHL Professor Anthony Zador to upgrade BARseq’s capabilities. What does that look like? Instead of thousands of neurons, BARseq can now map millions.
“We are focused on pushing BARseq forward. We want to make this easy for everybody to use, faster, more sensitive. Can we read out more information with it? With a much higher scale, you can start to answer different questions,” says Chen.
Research on Visual Cortex Using BARseq
The team began their search for answers in the brain’s visual cortex. Sight is one of the most common ways humans perceive the world. Information travels from the eyes to the visual cortex for processing. But what happens in the brain when the visual cortex’s neural inroads are cut or don’t form at all?
“People have known for a while that visual inputs are very important in shaping the brain,” Chen explains. “But we don’t know, at the exact cell-type resolution BARseq provides, what actually happens.”
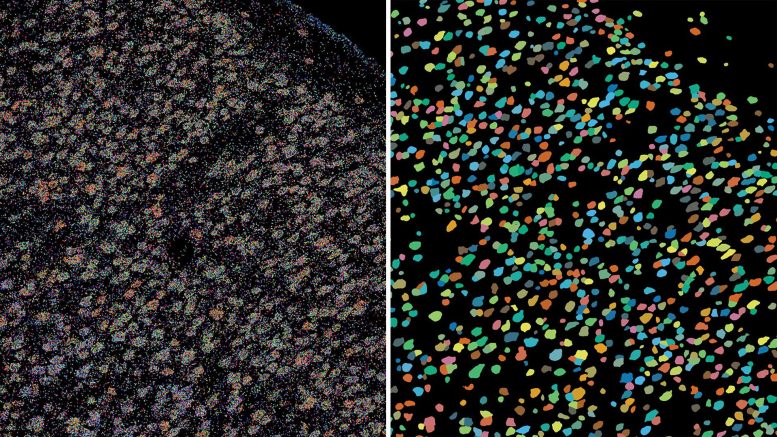
Left: Each colored dot in this image of the brain’s outer layer, or cortex, is an individual gene. Right: Using BARseq, scientists can see how genes are clustered and identify the corresponding neurons, the larger colored dots seen here. Credit: Chen lab/Allen Institute for Brain Science
The team used BARseq to map the brains of nine mice and traced gene expression in each mouse’s visual cortex. It’s the first time the technique has been used to map this many entire brains. Amazingly, the team found that if the mice went blind, the genes in the visual cortex started to look like those in neighboring cortical areas of the brain.
“The effects of losing vision were very broad,” Chen explains. “The visual cortex itself changes. It becomes more similar to the areas around it. There are still a lot of questions about how development controls this patterning.”
Chen is now working to expand BARseq’s capabilities even further. He and his team are using the technique to investigate how connections are wired in developing brains and how these connections evolve.
“Understanding how cortical areas are set up is the first step in understanding these connections,” he says. “But it’s not enough. We still need to discover how they progress during development. BARseq can bring us closer to that goal.”
Reference: “Whole-cortex in situ sequencing reveals input-dependent area identity” by Xiaoyin Chen, Stephan Fischer, Mara C. P. Rue, Aixin Zhang, Didhiti Mukherjee, Patrick O. Kanold, Jesse Gillis and Anthony M. Zador, 24 April 2024, Nature.
DOI: 10.1038/s41586-024-07221-6







Be the first to comment on "Pushing the Limits of Neuroscience: BARseq Is Mapping the Brain at a Million-Neuron Scale"