
The biosensor and reader are designed to be used at home or at a point of care. The system can transmit results wirelessly to physicians, patients, and patient’s caregivers and family. Credit: David Baillot/University of California San Diego
The next steps involve testing saliva and urine samples with the biosensor.
An international team of scientists has created a handheld, non-invasive device that can detect biomarkers for Alzheimer’s and Parkinson’s Diseases. This biosensor can also wirelessly send the findings to a laptop or smartphone.
The device underwent successful testing on in vitro samples from patients, demonstrating accuracy comparable to the most advanced current methods. The next phase involves experimenting with saliva and urine samples using this biosensor. Furthermore, there’s potential for adapting the device to detect biomarkers for various other medical conditions.
Researchers recently presented their findings in the journal Proceedings of the National Academy of Sciences.
The device relies on electrical rather than chemical detection, which researchers say is easier to implement and more accurate.
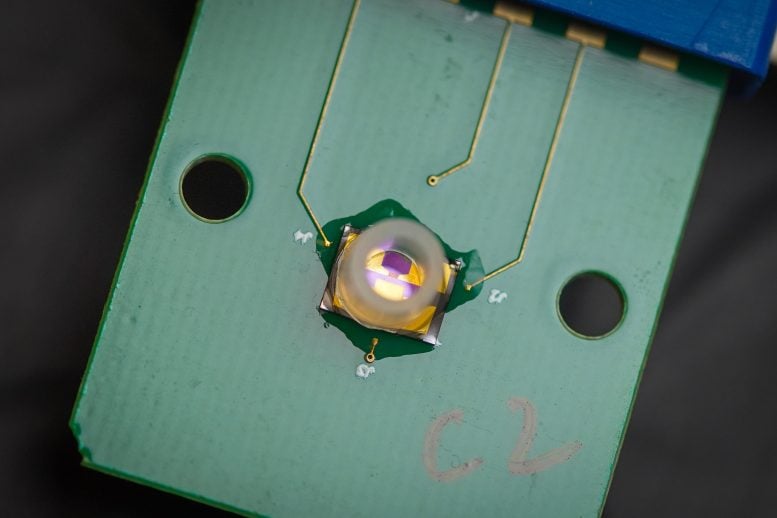
The biosensor consists of a chip with a highly sensitive transistor, made of a graphene layer that is a single atom thick and three electrodes–source and drain electrodes, connected to the positive and negative poles of a battery, to flow electric current, and a gate electrode to control the amount of current flow. Credit: David Baillot/University of California San Diego
“This portable diagnostic system would allow testing at-home and at point of care, like clinics and nursing homes, for neurodegenerative diseases globally,” said Ratnesh Lal, a bioengineering, mechanical engineering, and materials science professor at the UC San Diego Jacobs School of Engineering and one of the paper’s corresponding authors.
The Urgent Need for Early Detection
By the year 2060, about 14 million Americans will suffer from Alzheimer’s Disease. Other neurodegenerative diseases, such as Parkinson’s, are also on the rise. Current state-of-the-art testing methods for Alzheimer’s and Parkinson’s require a spinal tap and imaging tests, including an MRI. As a result, early detection of the disease is difficult, as patients balk at the invasive procedures. Testing is also difficult for patients who are already exhibiting symptoms and have difficulty moving as well as those who have no early access to local hospitals or medical facilities.
One of the prevailing hypotheses in the field, which Lal has focused on, is that Alzheimer’s Disease is caused by soluble amyloid peptides that come together in larger molecules, which in turn form ion channels in the brain.

A close up of the silicon well in the biosensor with the graphene-based transistor at the bottom. Credit: David Baillot/University of California San Diego
Lal wanted to develop a test that would be able to detect amyloid beta and tau peptides – biomarkers for Alzheimer’s – and alpha-synuclein proteins – biomarkers for Parkinson’s – non invasively, specifically from saliva and urine. He wanted to rely on electrical rather than chemical detection, as he believes it is easier to implement and more accurate. He also wanted to build a device that could wirelessly transmit the test results to the patient’s family and physicians. The device is the result of his three decades of expertise, as well as his collaboration with researchers globally, including those co-authors in this work from Texas and China.
“I am trying to improve quality of life and save lives,” he said.
To realize Lal’s vision, he and colleagues adapted a device they developed during the COVID pandemic to detect the spike and nucleoprotein proteins in the live SARS-CoV-2 virus, which they described in PNAS in 2022. That breakthrough had been made possible by chip miniaturization and by large-scale automation of biosensor manufacturing.
How the device is made and how it works
The device described in the 2023 PNAS study, consists of a chip with a high-sensitivity transistor, commonly known as a field effect transistor (FET). In this case, each transistor is made of a graphene layer that is a single atom thick (GFET, with the G standing for graphene) and three electrodes–source and drain electrodes, connected to the positive and negative poles of a battery, to flow electric current, and a gate electrode to control the amount of current flow.
Connected to the gate electrode is a single DNA strand, which serves as a probe that specifically binds to either amyloid beta, tau or synuclein proteins. The binding of these amyloids with their specific DNA strand probe, called an aptamer, changes the amount of current flow between the source and drain electrode. The change in this current or voltage is the signal used to detect the specific biomarkers, like amyloids or COVID-19 proteins.
The research team tested the device with brain-derived amyloid proteins from Alzheimer’s and Parkinson’s deceased patients. The experiments showed that the biosensors were able to detect the specific biomarkers for both conditions with great accuracy, on par with existing state-of-the-art methods. The device also works at extremely low concentrations, meaning that it needs small quantities for samples–down to just a few microliters.
In addition, the tests showed that the device performed well even when the samples analyzed contained other proteins. Tau proteins were more difficult to detect. But because the device looks at three different biomarkers, it can combine results from all three to arrive at a reliable overall result.
The technology has been licensed from UC San Diego to a biotechnology startup Ampera Life. Lal is the company’s chairman but does not receive financial support for his research from the company.
The next steps include testing blood plasma and cerebrospinal fluid with the device, then finally saliva and urine samples. The tests would take place in hospital settings and nursing homes. If those tests go well, Ampera Life plans to apply for FDA approval for the device, hopefully in the next five or six months. The ultimate goal is to have the device on the market in a year.
Reference: “In pursuit of degenerative brain disease diagnosis: Dementia biomarkers detected by DNA aptamer-attached portable graphene biosensor” by Tyler Andrew Bodily, Anirudh Ramanathan, Shanhong Wei, Abhijith Karkisaval, Nemil Bhatt, Cynthia Jerez, Md Anzarul Haque, Armando Ramil, Prachi Heda, Yi Wang, Sanjeev Kumar, Mikayla Leite, Tie Li, Jianlong Zhao and Ratnesh Lal, 13 November 2023, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2311565120
Funding for the research came from the National Institutes of Health, the University of California San Diego, and the Chinese Academy of Sciences. In addition, researchers used facilities that are a part of the NSF-funded UC San Diego Materials Research Science and Engineering Center.







As a ‘worse’ but not ‘worst’ case lay scenario with a family history of both food allergies not yet recognized or researched by mainstream medicine and dementia, in early 2010 and mysteriously (to my doctor), seriously ill again I began testing my own saliva and urine non-invasively both non-electronically and with a non-wireless electronic device for what I postulate to be a reliable very early onset marker for Alzheimer’s Disease (minimally); pH. The published healthy normal for pH is said to be slightly alkaline, about 7.3. The pH testing strips I found online cost about six cents each by the hundreds and the electronic pH tester cost about $40. With the electronic pH tester requiring cleaning and a standardizing solution I quickly switched to the pH testing strips, for convenience and immediate results. A decade and thousands of email comments to health professionals later, mainstream medicine still fails to make the connection between my (Dr. Arthur F. Coca’s; by 1935) kind of chronic very, very mild nearly subclinical non-IgE-mediated food allergies and acidic (perhaps myelin sheath eroding) blood, which can result in chronic acidic saliva and urine.