
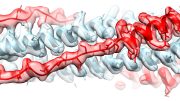
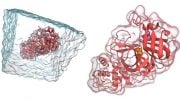
On the left panel, Salvia divinorum plant. On the right panel, location of drug salvinorin A in the kappa opioid receptor. Credit: Roth lab, UNC-Chapel Hill
A team of researchers have determined the structure of the kappa-opioid receptor, KOR, and give details about how salvinorin A and other drugs interact with it in a newly published paper. The findings may lead to the design of medicines that either activate or block KOR to benefit medical patients suffering from chronic pain, cocaine addiction and other diseases.
At the molecular level, drugs like salvinorin A (the active ingredient of the hallucinogenic plant Salvia divinorum) work by activating specific proteins, known as receptors, in the brain and body.
Salvinorin A, the most potent naturally occurring hallucinogen, is unusual in that it interacts with only one receptor in the human brain — the kappa opioid receptor (KOR). Scientists know of four distinct types of opioid receptors, but until now the structure of the ‘salvia receptor’, and the details about how salvinorin A and other drugs interact with it, was a mystery.
In a research paper published March 21 in the journal Nature, scientists from the University of North Carolina at Chapel Hill, Scripps Research Foundation and two other institutions revealed the first-ever glimpse of the complete structure of the KOR. The finding could accelerate the development of new drugs to treat addiction, depression, anxiety, chronic pain, and many other conditions.
“Once we see the structure of the receptor, it becomes easier for us to develop drugs that target the receptor in ways that might be beneficial for medical therapy,” said Bryan Roth, MD, PhD, the Michael Hooker Distinguished Professor of Pharmacology at UNC and one of the paper’s authors. “Drugs that block the receptor are potentially useful for treating a number of serious illnesses including chronic pain, cocaine addiction, and other diseases.”
The KOR is responsible for the action of drugs that affect human consciousness, awareness of pain, and mood. The KOR is the only receptor that binds salvinorin A — the active ingredient of the widely-abused hallucinogenic plant Salvia divinorum (also known as ‘Magic mint’, ‘Salvia’ and so on). Salvia use has surged among teenagers and young adults with more than 5 percent reporting usage in the past year (Source: National Institute on Drug Abuse InfoFacts).
Knowing KOR’s structure offers insights into how salvia and other drugs work. The finding also could help scientists design medicines that either activate or block KOR to benefit patients.
The research team crystallized KOR using JDTic, a drug currently in early-stage human trials; JDTic keeps KOR in an inactive state and blocks the actions of salvinorin A. In studies using animal models, JDTic has shown promise for treating cocaine and nicotine addiction, depression, and anxiety.
The downside of JDTic and similar drugs targeting KOR is that they can exert their actions for weeks, whereas most prescribed medicines are cleared within 18-24 hours. “Now that we have the [KOR] structure, it opens up the possibility for us to make drugs that have the same action as JDTic but have better pharmaceutical properties,” said Roth.
The research also resolves longstanding scientific debates about how drugs bind to KOR. The study found that compared to other receptors, the binding site on KOR is enormous, allowing drugs to bind to it in more than one way.
In addition, Roth said the research could help scientists develop drugs that activate KOR in the body without affecting the brain, which could be useful for treating chronic pain, kidney problems, and many other disorders.
Reference: “Structure of the human κ-opioid receptor in complex with JDTic” by Huixian Wu, Daniel Wacker, Mauro Mileni, Vsevolod Katritch, Gye Won Han, Eyal Vardy, Wei Liu, Aaron A. Thompson, Xi-Ping Huang, F. Ivy Carroll, S. Wayne Mascarella, Richard B. Westkaemper, Philip D. Mosier, Bryan L. Roth, Vadim Cherezov and Raymond C. Stevens, 21 March 2012, Nature.
DOI: 10.1038/nature10939
Co-authors include Eyal Vardy and Xi-Ping Huang at UNC-Chapel Hill; Huixian Wu, Daniel Wacker, Vsevolod Katritch, Mauro Mileni, Gye Won Han, Wei Liu, Aaron A. Thompson, Vadim Cherezov and Raymond C. Stevens from the Scripps Research Institute; F. Ivy Carroll and S. Wayne Mascarella from the Research Triangle Institute; and Richard B. Westkaemper and Philip D. Mosier from Virginia Commonwealth University.
The study was supported by funding from the National Institutes of Health.





Be the first to comment on "Complete Structure of the “Salvia Receptor” Revealed"